Electron Dot Diagram For Chlorine
Notable exceptions to the octet rule. Lewis structures or electron dot structures depict the bonds between atoms of a molecule and any unbonded electron pairs.
Electron Dot Diagram For Hydrogen Chloride Astonishing Covalent Bond
Lewis structures or electron dot structures.
Electron dot diagram for chlorine. For electron dot diagrams this symbol represents the nucleus and all of the electrons of the atom except the outermost electrons. A lewis structure is a type of shorthand notation. For the cl 2 lewis structure there are a total of 14 valence electrons available.
Search the site go. In an electron dot diagram this symbol represents the nucleus and the ten electrons in the first two energy levels. The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding.
Since it is in group 7 it will have 7 valence electrons. In other words if every element in group 1a has 1 valence electron then every lewis electron dot diagram would have one single dot. Check the formal charges to make sure you have the best lewis structure.
Since the lewis electron dot diagrams are based on the number of valence electrons it would hold true that the elements in the same group would have the same electron dot diagram. For example when two chlorine atoms each with 7 valence electrons come together to form a diatomic chlorine molecule the lewis structure shows that there will be a sharing of two electrons between the two chlorine atoms which allows both chlorine to be surrounded by 8 electrons. S and p sometimes have more than 8 val.
Each dot represents a valence electron each line represents a bonded pair of electrons and each cl represents a chlorine atom. Here is how to draw them. H only needs 2 valence electrons.
The symbol for chlorine is cl. The lewis dot structure for cl2 the chemical formula for chlorine gas is written with two cl symbols each of which is surrounded by three pairs of dots connected by a single line. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
After that i draw the lewis dot structure for chlorine cl. Chlorine is in group 17 sometimes called group vii or 7a. When two chlorine atoms form a chlorine molecule they share one pair of electrons.
See the big list of lewis structures transcript. So 4 2 n 2 o xeo 3. Be and b dont need 8 valence electrons.
Ok were going to draw the dot structure for chlorine gasa poisonous green gas.
 Covalent Bonding Lewis Structures
Covalent Bonding Lewis Structures
 Write Electron Dot Structure For Chlorine At No 17 And Calcium
Write Electron Dot Structure For Chlorine At No 17 And Calcium
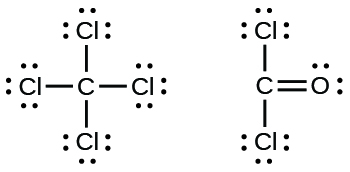 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
Electron Dot Diagram For S Elegant Electron Dot Diagram For Chlorine
Lewis Dot Structure Of Clo2 Chlorite Ion Action News Abc Action
 Draw An Electronic Dot Structure Of Chlorine Brainly In
Draw An Electronic Dot Structure Of Chlorine Brainly In
 Dot Diagram For Chlorine Best Wiring Library
Dot Diagram For Chlorine Best Wiring Library
 Clo Lewis Dot Diagram Wiring Diagram Specialties
Clo Lewis Dot Diagram Wiring Diagram Specialties
 Vocabulary 1 Valence Shell 2 Valence Electrons 3 Anion 4 Cation
Vocabulary 1 Valence Shell 2 Valence Electrons 3 Anion 4 Cation
Chlorine Dot Diagram Awesome How To Draw The Lewis Dot Structure For
 Lewis Dot Structures Study Com
Lewis Dot Structures Study Com
Sodium Chlorine Diagram Block And Schematic Diagrams 28669724632
Multimedia Represent Bonding With Lewis Dot Diagrams Chapter 4
What Is The Formation Of Kcl With An Electron Dot Structure Quora
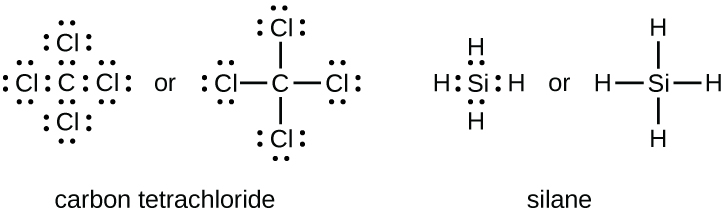 Dot Diagram Element Chlorine Wiring Diagram
Dot Diagram Element Chlorine Wiring Diagram
Chlorine Dot Diagram Best Chlorine Electron Dot Symbol Wiring



0 Response to "Electron Dot Diagram For Chlorine"
Post a Comment