Examine The Following Phase Diagram And Determine What Phase Exists At Point D
Ammonias unusually high melting point is the result of adipole dipole forces. Neon condenses due to a dipole dipole forces.
 A Possible Four Phase Coexistence In A Single Component System
A Possible Four Phase Coexistence In A Single Component System
Examine the phase diagram for the substance bogusium bo and select the correct statement.
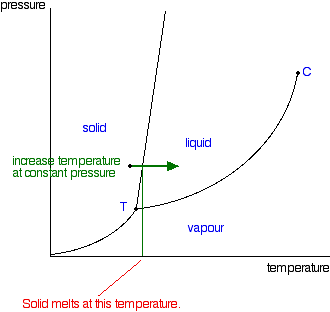
Examine the following phase diagram and determine what phase exists at point d. Examine the following phase diagram and determine. Point b in this phase diagram represents the only combination of temperature and pressure at which a pure substance can exist simultaneously as a solid a liquid and a gas. Liquiddsolidesupercritical fluid 3 8.
B the triple point for bo is at a higher temperature than the melting point for bo. Calculuate the temperature at which its vapor pressure is exactly half of that at its normal boiling point. Chapter 12 consider the following phase diagram and identify the process occurring as one goes from point c to point d.
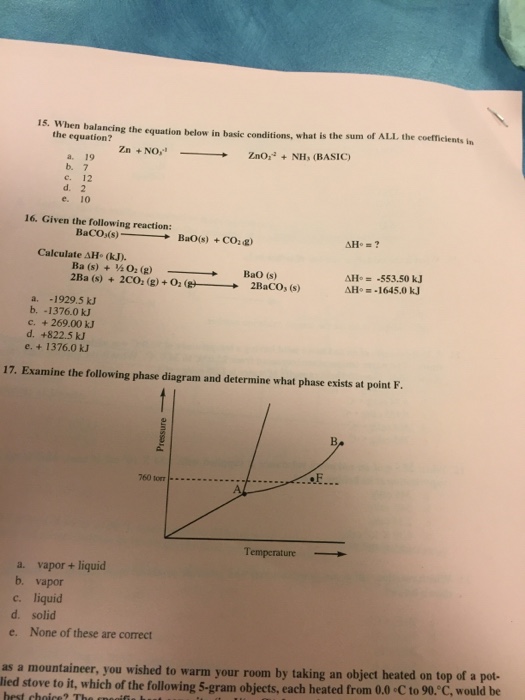
Examine the following phase diagram and determine what phase exists at point f. A bos has a lower density than bol. Examine the following phase diagram and determine what phase exists at point f.
Examine the following phase diagram and determine what phase exists at point favapor liquidb. Increasing temperature with a phase change from solid to vapor examine the following phase diagram and determine what phase exists at point f. A vapor liquid.
Examine the following phase diagram and identify the feature represented by point a and point b. B london dispersion forces. C bo changes from a solid to a liquid as one follows the line from c to d.
The heat of vaporization for either is 2669 kjmol. Vapor which of the following intermolecular forces is the weakest. Examine the phase diagram for the substance bogusium bo and select the correct statement.
This preview has intentionally blurred sections. White fall 2013 10. A dispersion forces b dipole dipole interactions c dipole.
Examine the following phase diagram and identify the feature represented by point a. Examine the following phase diagram and identify the feature represented by point a. A vapor liquid b vapor c liquid d solid e supercritical fluid 4.
Examine the phase diagram for the substance bogusium bo and select the correct statement. Amelting point bcritical point ctriple point dsublimation point eboiling point 9. C bo changes from a solid to a liquid as one follows the line from c to d.
It is therefore called the triple point of the substance and it represents the only point in the phase diagram in which all three states are in equilibrium. The normal boiling point of ether is 3078 k. Sign up to view the full version.
B the triple point for bo is at a higher temperature than the melting point for bo. See question 10 image a bos has a lower density than bol.
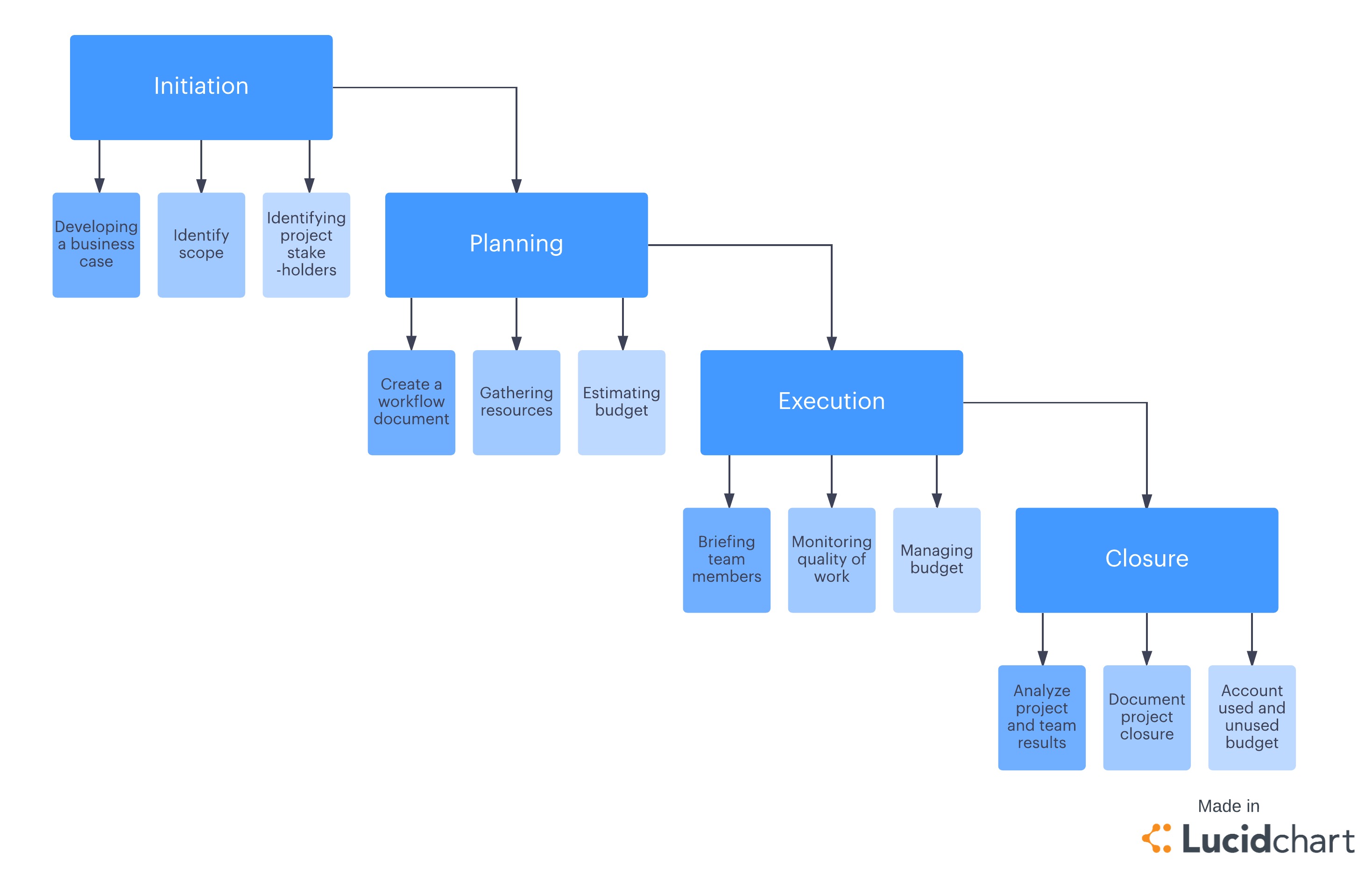 The 4 Phases Of The Project Management Life Cycle Lucidchart
The 4 Phases Of The Project Management Life Cycle Lucidchart
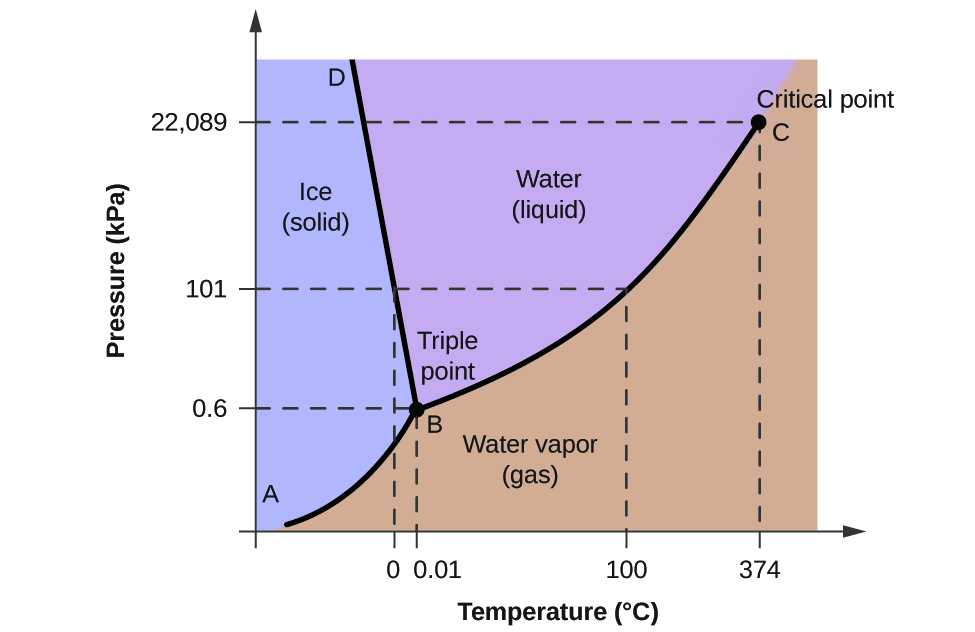 10 4 Phase Diagrams Chemistry Libretexts
10 4 Phase Diagrams Chemistry Libretexts
 Phase State Of Matter Britannica Com
Phase State Of Matter Britannica Com
 Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09
Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09
 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
 Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09
Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09
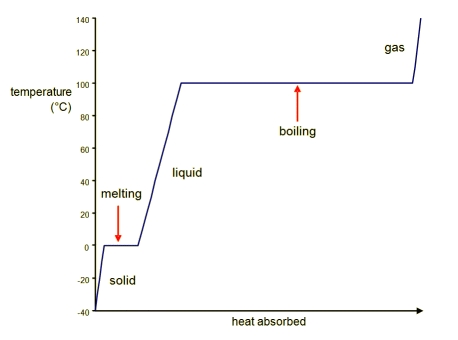 Phase Changes Boundless Chemistry
Phase Changes Boundless Chemistry
 A Possible Four Phase Coexistence In A Single Component System
A Possible Four Phase Coexistence In A Single Component System
 Phase Changes Boundless Chemistry
Phase Changes Boundless Chemistry
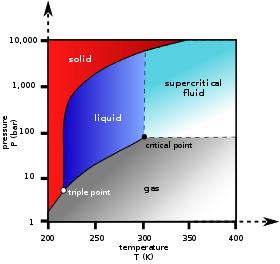
 10 1 Intermolecular Forces Chemistry
10 1 Intermolecular Forces Chemistry

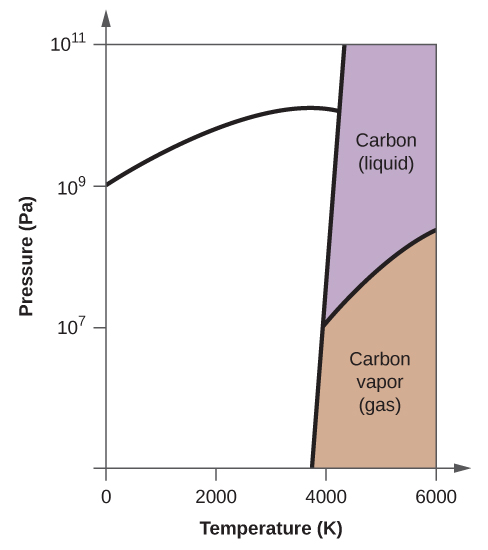

0 Response to "Examine The Following Phase Diagram And Determine What Phase Exists At Point D"
Post a Comment