Electron Dot Diagram For C
When examining chemical bonding it is necessary to keep track of the valence electrons of each atom. Electron dot structure valence electrons are represented by dots placed around the chemical symbol.
 Lewis Dot Structure For Ci4 Carbon Tetraiodide Youtube
Lewis Dot Structure For Ci4 Carbon Tetraiodide Youtube
Lewis electron dot diagrams for ions have less for cations or more for anions dots than the corresponding atom.
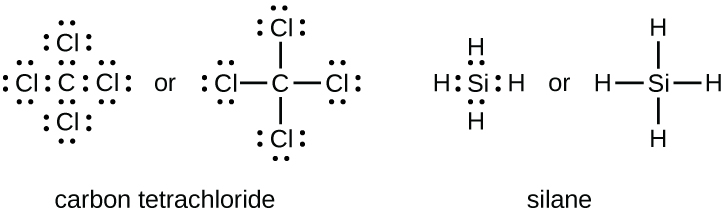
Electron dot diagram for c. Procedure for positively charged ions nh 4 use the same procedure as outlined above then remove one electron per postive charge as needed to avoid expanded octets. Explain why the first two dots in a lewis electron dot diagram are drawn on the same side of the atomic symbol. What is an electron dot diagram.
Lewis electron dot diagrams for ions have fewer for cations or more for anions dots than the corresponding atom. Write the lewis structure for a molecule of the compound. Electrons are placed up to two on each side of the elemental symbol for a maximum of eight which is the number of electrons in a filled s and p shell.
A beryllium atom with two valence electrons would have the electron dot diagram below. The final lewis structure for carbonate ion is. An electron dot diagram is a method of writing the chemical symbol of an element by surrounding it with dots to indicate the number of valence electrons.
A lewis structure can be drawn for any covalently bonded molecule as well as coordination compounds. Electron dot diagrams sometimes called lewis dot diagrams were first used by gilbert n. How about c and h.
When using this procedure for positively charged ions it may be necessary to have some atoms with expanded octets nitrogen in. A step by step explanation of how to draw the lewis dot structure for c carbon. A compound with a molar mass of about 42 gmol contains 857 carbon and 143 hydrogen by mass.
Exercises explain why the first two dots in a lewis electron dot diagram are drawn on the same side of the atomic symbol. These diagrams are used as a shorthand notation to show the number of valence electrons in an atom. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the elements symbol.
A compound with a molar mass of about 28 gmol contains 857 carbon and 143 hydrogen by mass. Write the lewis structure for a molecule of the compound. Valence electrons are found in an atoms outer shell and are the ones involved in chemical reactions.
Lewis structures also known as lewis dot diagrams lewis dot formulas lewis dot structures electron dot structures or lewis electron dot structures leds are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Electron dot structures helpful tools in thinking about bonding. I show you where carbon is on the periodic table and how how to determine how many valence electrons it has.
 What Is The Difference Between A Lewis Dot Symbol And A Lewis
What Is The Difference Between A Lewis Dot Symbol And A Lewis
Electron Dot Diagram For Carbon Monoxide Michaelhannan Co
Lewis Dot Structures And Covalent Bonding Sas
 Electron Dot Formula Electron Dot Formula For H2o Cs2 O2 Hcl
Electron Dot Formula Electron Dot Formula For H2o Cs2 O2 Hcl
 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry

Carbon Electron Dot Diagram Synthesis Amphiphilic Carbon Dot
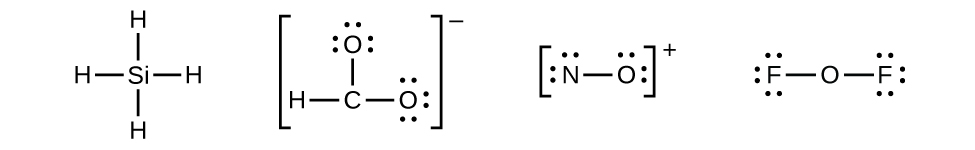 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
 Carbon Has Four Valence Electrons And Oxygen Has Six Valence
Carbon Has Four Valence Electrons And Oxygen Has Six Valence
 Electron Dot Formula Electron Dot Formula For H2o Cs2 O2 Hcl
Electron Dot Formula Electron Dot Formula For H2o Cs2 O2 Hcl
 Lewis Dot Diagrams Ems Chemistry Part 2
Lewis Dot Diagrams Ems Chemistry Part 2
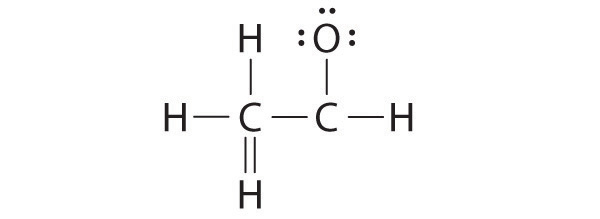 Lewis Structures And Covalent Bonding
Lewis Structures And Covalent Bonding
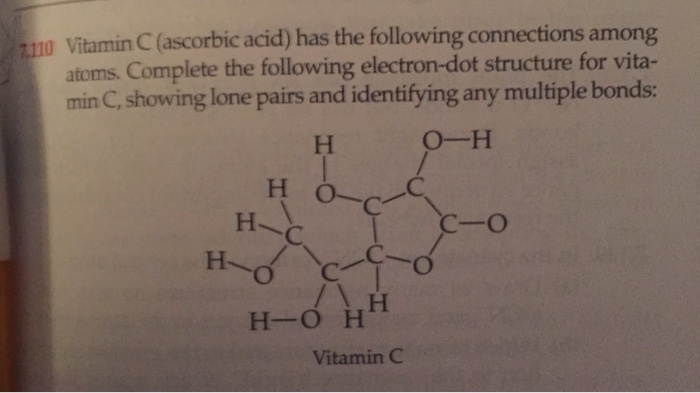
 Study The Electron Dot Diagrams For Lithium Carbon Fluorine And Neon
Study The Electron Dot Diagrams For Lithium Carbon Fluorine And Neon
Carbon Electron Dot Diagram Synthesis Amphiphilic Carbon Dot
 Chemistry Notation Orbital And Lewis Dot Shmoop Chemistry
Chemistry Notation Orbital And Lewis Dot Shmoop Chemistry
Electron Dot Diagram For Nitrogen Lewis Structure Hydrogen And


0 Response to "Electron Dot Diagram For C"
Post a Comment