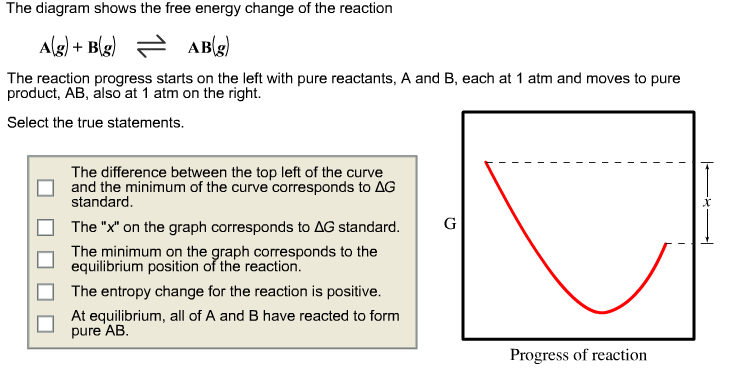
The Diagram Shows The Free Energy Change Of The Reaction
Show transcribed image text the diagram shows the free energy change of the reaction ag bg rightarrow cg the reaction progress starts on the left with pure reactants a and b. 2 calculate the partial pressure of ag at equilibrium.
 W2018 Bis2a Lecture10 Reading Biology Libretexts
W2018 Bis2a Lecture10 Reading Biology Libretexts
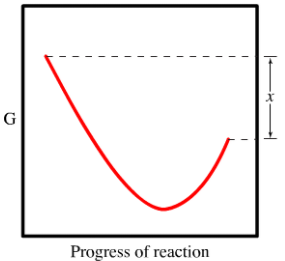
The diagram shows the free energy change of the reaction ab the reaction progress starts on the left with pure reactants a and b each at 1 atm and moves to pure product ab also at 1 atm on the right.
The diagram shows the free energy change of the reaction. When talking about thermal dynamics and kinetics one of our best friends is going to be the free energy diagram. A reaction coordinate diagram shows the energy changes that take place in each of the steps of the mechanism. Show transcribed image text the diagram shows the free energy change of the reaction the reaction progress starts on the left with pure reactants a and b.
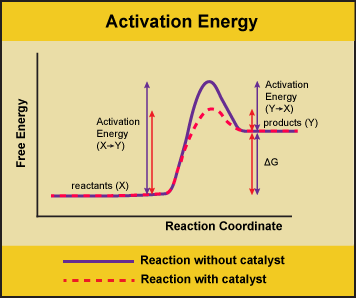
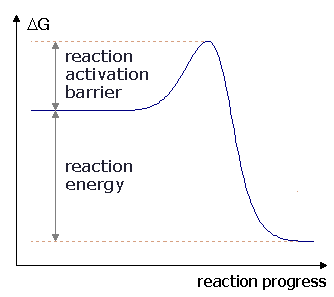
However for endothermic reactions the reactants are drawn below the products. Each at 1 atm and moves to pure product c also at 1 atm on the right. Energy diagrams depict the reaction progress versus energy.
The entropy change for the reaction is positive. For exothermic reactions the reactants are drawn above the products because their energy is greater. A what is the significance of the minimum in the plotb what does the quantity x shown on the right side of the diagram represent.
Ag in equilibrium with bg at 25 degrees c. Reaction coordinate diagrams the mechanism of a reaction describes the various steps that are believed to occur as reactants are converted into products. The reason free energy diagrams are important is because theyre going to serve as a summary of the thermal dynamics and kinetics of a reaction.
Free energy and equilibrium. Select the true statements. Catalysts lower activation energy so they decrease the size of the hump within the diagram itself.
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. In a reaction coordinate diagram the total energy of all species is plotted. It also shows the effect of a catalyst on the forward and reverse activation energy.
Spontaneous nonspontaneous q k q k. Place the statements in the appropriate place on the diagram. The accompanying diagram shows how the free energy g changes during a hypothetical reaction ag bg cg on the left are pure reactants each at 1 atm and on the right is the pure product also at 1 atm.
Assume that g subscript a 8850 jmol and g subscript b 12490 jmol 1calculate the value of the equilibrium constant for this reaction. Each at 1 atm and moves to pure product ab also at 1 atm on the right. The diagram shows the free energy change of the reaction ag bg cg.
At equilibrium all of a and b have reacted to form pure ab. The reaction progress starts on the left with pure reactants a and b each at 1 atm and moves to pure product c also at 1 atm on the right. So its going to be essential that we learn how to interpret these correctly.
 Quia Ap Chapter 8 An Introduction To Metabolism Detailed
Quia Ap Chapter 8 An Introduction To Metabolism Detailed
 Solved The Diagram The Free Energy Of The Reaction A G
Solved The Diagram The Free Energy Of The Reaction A G
Water Ionization The Ionic Product Kw Of Water And Ph
Notes Chapter 17 Equilibrium Kinetics
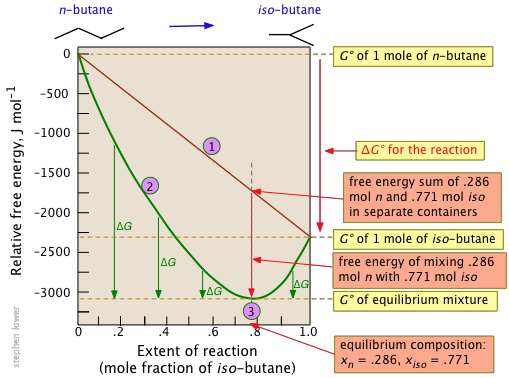
 The Relationship Between Free Energy And The Equilibrium Constant
The Relationship Between Free Energy And The Equilibrium Constant
 Shows The Change In Free Energy A And Log Likelihood B For The
Shows The Change In Free Energy A And Log Likelihood B For The
 Free Energy And Equilibrium Chemistry Libretexts
Free Energy And Equilibrium Chemistry Libretexts
How Much Energy Is Released In Atp Hydrolysis
How Much Energy Is Released In Atp Hydrolysis
Factors That Affect The Rate Of Reactions Introductory Chemistry
Metamorphic Petrology Geology 102c
 Chemical Reaction Definition Equations Examples Types
Chemical Reaction Definition Equations Examples Types
 Solved The Accompanying Diagram Shows How The Free Energy G
Solved The Accompanying Diagram Shows How The Free Energy G
 Shows The Change In Free Energy A And Log Likelihood B For The
Shows The Change In Free Energy A And Log Likelihood B For The
 The Diagram Shows The Free Energy Change O Clutch Prep
The Diagram Shows The Free Energy Change O Clutch Prep
Energy Enzymes And Catalysis Problem Set

0 Response to "The Diagram Shows The Free Energy Change Of The Reaction"
Post a Comment