Lewis Dot Diagram For Co
Put one electron pair in each bond 4. A brief tutorial on drawing lewis dot structures.
 Co Lewis Structure How To Draw The Dot Structure For Co Youtube
Co Lewis Structure How To Draw The Dot Structure For Co Youtube
For the co 2 lewis structure there are a total of 16 valence electrons available.
:max_bytes(150000):strip_icc()/ICl3_LD-56a12a2b3df78cf77268034c.png)
Lewis dot diagram for co. In the formal way we find how many electrons we have step 1 how many each atom needs step 2 how many of those are bonding step 3 4 and how many are lone pairs step 5. Lewis dot of carbon monoxide. We will use three molecules co 2 co 3 2 and nh 4 as our examples on this guided tour of a simple method for drawing lewis dot structureswhile this algorithm may not work in all cases it should be adequate the vast majority of the time.
70 more lewis dot structures. Fill outer atoms with electrons 5. A lewis structure is a graphic representation of the electron distribution around atoms.
Produced from the incomplete combustion of hydrocarbons. The key is to understand the steps and practice. In the winter it is important that furnaces have access to an ample supply of oxygen in the air.
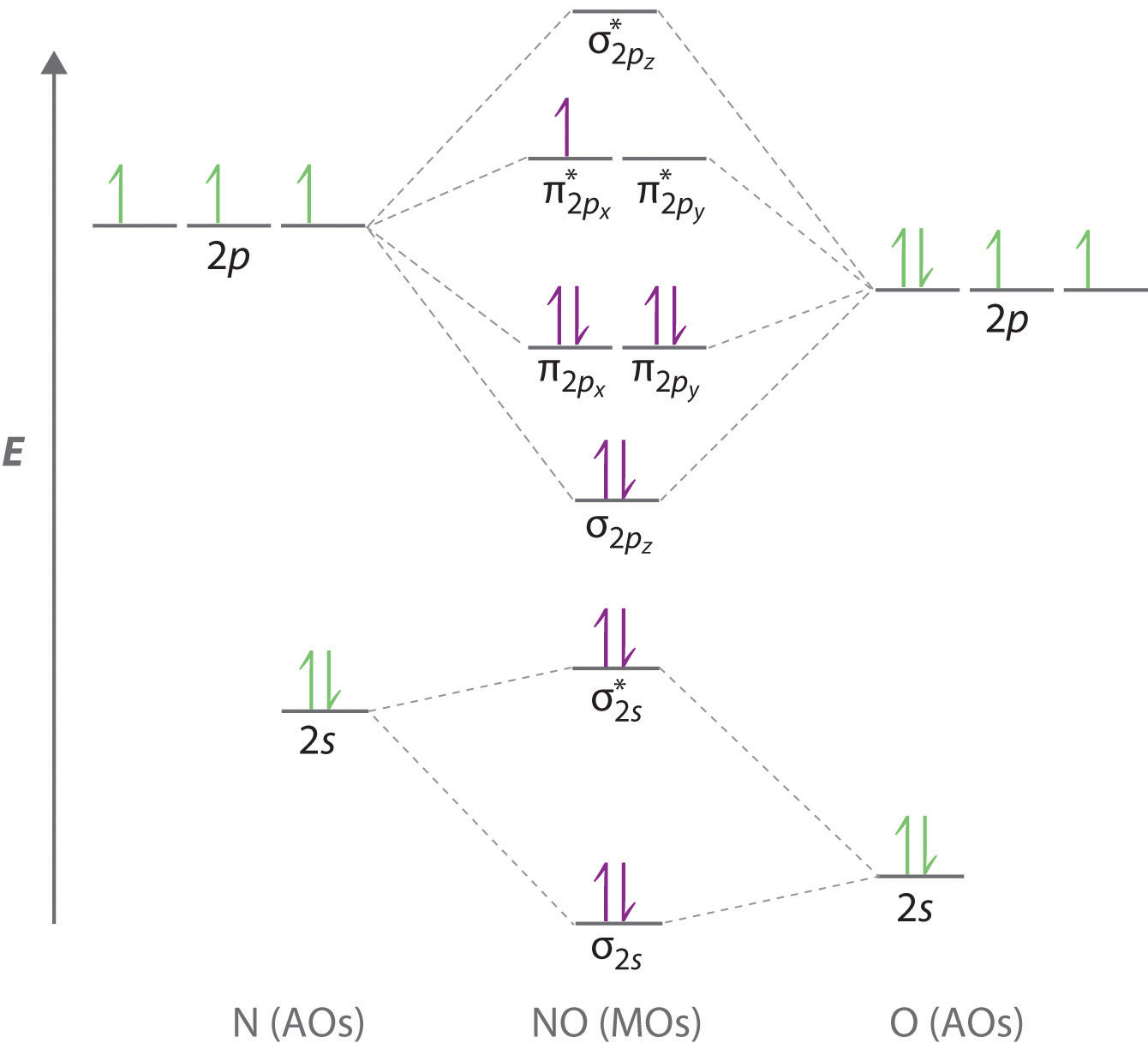
The notable exceptions are the compounds cn cyanide and co carbon monoxide which the bond is polar due to the unequal sharing of electrons between the two atoms in the structure. Put least electronegative atom in centre 3. The lewis structure lewis dot diagram for co.
The lewis structure for co has 10 valence electrons. After determining how many valence electrons there are in co place them around the central atom to complete the octets. In the earths atomsphere it is considered a greenhouse gas.
Every chemistry student has to learn how to draw lewis dot structures. For the co lewis structure. A lewis structure also helps to make a prediction about the geometry of a molecule.
Co 2 is a clear heavier than air gas. In the co 2 lewis structure carbon is the least electronegative element. It is a colorless odorless tasteless gas that highly toxic to humans.
How the molecule might react with other molecules. Therefore it is put in the center of the dot structure. The physical properties of the molecule like boiling point surface tension etc.
This info can then be used to determine the lewis dot structure. There is an easy way and a formal way to draw the lewis structure of co carbon monoxide. For the co lewis structure calculate the total number of valence electrons for the co molecule.
The shape of a molecule. The structures do not look like the lewis dot structure for co2 where the carbon does not have any lone pair electrons on the carbon. Lewis structures are important to learn because they help us predict.
The reason for learning to draw lewis structures is to predict the number and type of bonds that may be formed around an atom.
 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
:max_bytes(150000):strip_icc()/ICl3_LD-56a12a2b3df78cf77268034c.png) The Octet Rule Explanation In Chemistry
The Octet Rule Explanation In Chemistry
 Lewis Dot Structure Of Co3 2 Carbonate Ion Youtube
Lewis Dot Structure Of Co3 2 Carbonate Ion Youtube
 Dot Diagram Co Wiring Diagram Database
Dot Diagram Co Wiring Diagram Database
 Co Electron Dot Diagram Wiring Diagram Database
Co Electron Dot Diagram Wiring Diagram Database
 9 2 The Vsepr Model Chemistry Libretexts
9 2 The Vsepr Model Chemistry Libretexts
 Lewis Diagram Ccl4 Best Wiring Library
Lewis Diagram Ccl4 Best Wiring Library
Methane Electron Dot Diagram Prettier Methane Atom Scaffold Diagram
 Ch3oh Dot Structure Arlenmuniz S Blog
Ch3oh Dot Structure Arlenmuniz S Blog

Co Ordinate Dative Covalent Bonding

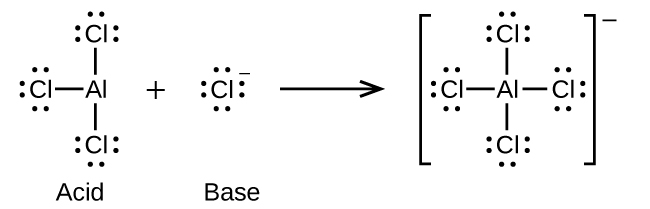 15 2 Lewis Acids And Bases Chemistry
15 2 Lewis Acids And Bases Chemistry
Electron Dot Diagram For Co Awesome Formal Charge Formula
 Electron Dot Formula Electron Dot Formulas Formulas Tutorvista Com
Electron Dot Formula Electron Dot Formulas Formulas Tutorvista Com
 What Would Be The Electron Dot Structure Of Molecule Of Sulphur
What Would Be The Electron Dot Structure Of Molecule Of Sulphur
It Has Three Unpaired Electrons Each Of Which Can Make A Bond By
 Carbonate Ion Compu Ibmdatamanagement Co
Carbonate Ion Compu Ibmdatamanagement Co
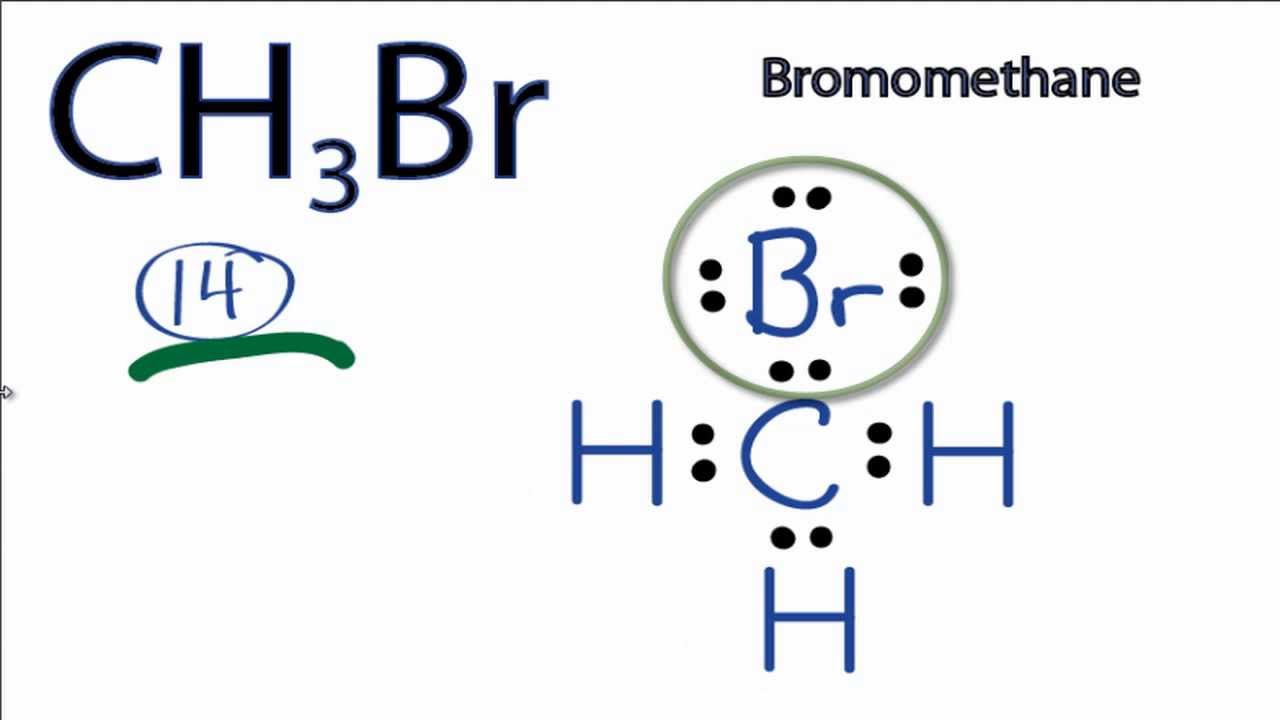 Lewis Diagram Chbr3 18 6 Stromoeko De
Lewis Diagram Chbr3 18 6 Stromoeko De
0 Response to "Lewis Dot Diagram For Co"
Post a Comment