Lewis Dot Diagram For Sulfur
A d b y c o d e f e l l o w s. The lewis dot structure for sulfur is an s with 6 dots which stand for its six valence electrons.
 Krypton Dot Diagram 2 Artatec Automobile De
Krypton Dot Diagram 2 Artatec Automobile De
So the resonance structure on the left and the resonance structure on the right and some people disagreed with me and said thats not the dot structure for sulfur dioxide.

Lewis dot diagram for sulfur. The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons. A lewis electron dot diagram a representation of the valence electrons of an atom that uses dots around the symbol of the element. The lewis structure for so 3 2 is requires you to place more than 8 valence electrons on sulfur s.
The lewis dot structure for sulfur includes the upper case letter s the elements chemical symbol surrounded by two sets of paired dots and two single dots evenly spaced around the letter. A step by step explanation of how to draw the lewis dot structure for s sulfur. The lewis structure for so 4 2 is requires you to place more than 8 valence electrons on sulfur s.
Drawing the lewis structure for so 3 2. Exercises explain why the first two dots in a lewis electron dot diagram are drawn on the same side of the atomic symbol. How can you determine the lewis dot structure of hydrogen sulfide h2s.
Voiceover in the previous video we looked at the dot structure for sulfur dioxide and i drew out two resonance structures. At code fellows you can graduate with two years of relevant industry experience in just 20 weeks. Want to become a software developer in seattle.
How can you determine the lewis dot structure for sulfur. You might think youve got the correct lewis structure for so 3 at first. I show you where sulfur is on the periodic table and how to determine how many valence electrons sulfur has.
You might think youve got the correct lewis structure for so 4 at first. Remember sulfur is in period 3 and can hold more than 8 valence electrons. Or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
Remember sulfur is in period 3 and can hold more than 8 valence electrons. Lewis electron dot diagrams for ions have fewer for cations or more for anions dots than the corresponding atom.
 More Bonding Quick Overview Of Ionic Bonding Metallic Bonding
More Bonding Quick Overview Of Ionic Bonding Metallic Bonding
Lewis Electron Dot Diagrams Introductory Chemistry 1st Canadian
 Sulfur Lewis Structure Schematic Diagram
Sulfur Lewis Structure Schematic Diagram
 File Lewis Dot S Svg Wikimedia Commons
File Lewis Dot S Svg Wikimedia Commons
 What Are The Resonance Structures For So 2 Socratic
What Are The Resonance Structures For So 2 Socratic
Images Of Sulfur Lewis Dot Structure Golfclub
8 1 Chemical Bonds Lewis Symbols And The Octet Rule Chemistry
 Lewis Dot Structure Sulfur Tetrabromide
Lewis Dot Structure Sulfur Tetrabromide
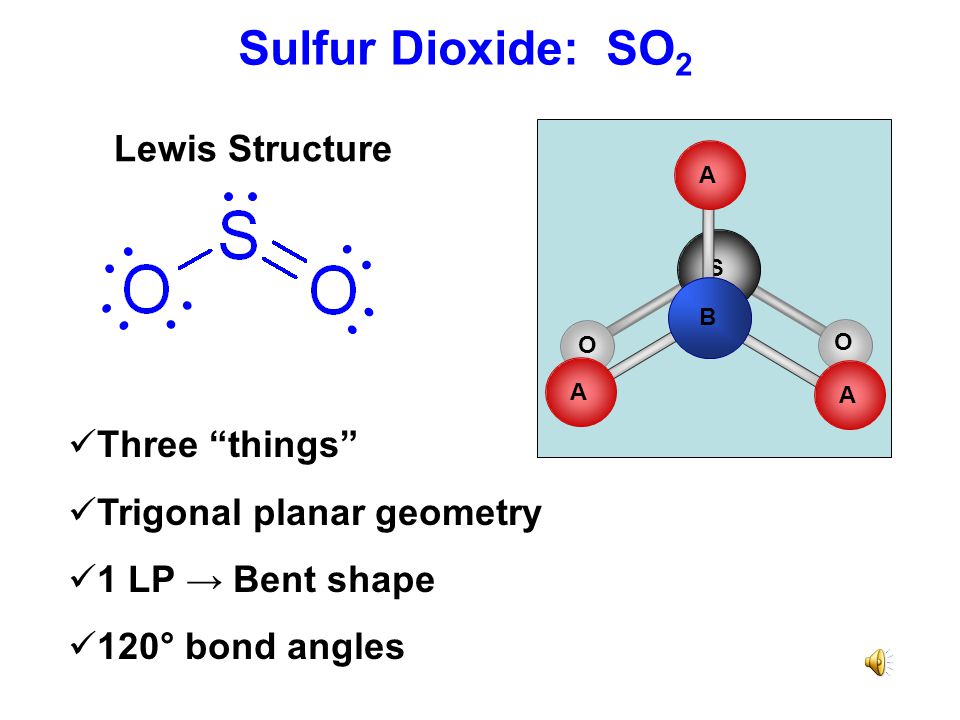 Lewis Dot Diagram For Sulphur Dioxide Nemetas Aufgegabelt Info
Lewis Dot Diagram For Sulphur Dioxide Nemetas Aufgegabelt Info
 Cs2 Dot Diagram Fresh Electron Dot Diagram Sulfur Cute H2s
Cs2 Dot Diagram Fresh Electron Dot Diagram Sulfur Cute H2s
Chem4kids Com Sulfur Orbital And Bonding Info
 In The Molecule H 2s Four Electron Groups Around The Sulfur Atom
In The Molecule H 2s Four Electron Groups Around The Sulfur Atom
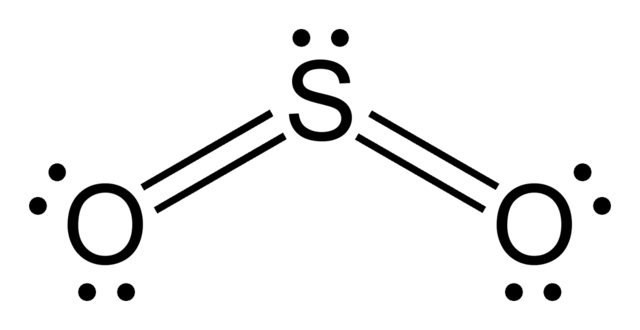 Electron Dot Diagram For Sulfur Molecular Bonding Solved Draw The
Electron Dot Diagram For Sulfur Molecular Bonding Solved Draw The
Lewis Diagram For Ch4 Inspirational Methane Ch4 Lewis Dot Structure
 Lewis Dot Structure For Sulfur Tetrafluoride Awesome Vsepr Geometry
Lewis Dot Structure For Sulfur Tetrafluoride Awesome Vsepr Geometry

Sulfur Electron Dot Diagram Awesome Lewis Diagram Sulfur Schematic

0 Response to "Lewis Dot Diagram For Sulfur"
Post a Comment