Electron Distribution Diagram Of Carbon
How many valence electrons does carbon have. The skeleton may have double bonds which can vary in location.
:max_bytes(150000):strip_icc()/hydrogenatom-58b6029c3df78cdcd83d98bb.jpg) Atom Diagrams Electron Configurations Of The Elements
Atom Diagrams Electron Configurations Of The Elements
How to write electron configurations and.
:max_bytes(150000):strip_icc()/magnesiumatom-58b6026b5f9b5860464c7467.jpg)
Electron distribution diagram of carbon. C 12 c 13 and c 14 are isotopes of the element carbon. Greg robsoncc by 20. Sodium greg robsoncc by 20.
The electron shells are shown moving outward from the nucleus. In atomic physics and quantum chemistry the electron configuration is the distribution of electrons of an atom or molecule or other physical structure in atomic or molecular orbitals. How to write the electron configuration for carbon.
Carbon skeletons vary in length. The remaining two electrons will go in the 2p orbital. List here the types of skeletons that can be formed.
Carbon chains form skeletons. It is essential that you know the answers to these questions. What type of bonds does it form with other elements.
4 quantum numbers electron configuration orbital diagrams. Make an electron distribution diagram of carbon. Note that the last term in the carbon electron configuration will be 1s2 2s2 2p2.
For example the electron configuration of the neon atom is 1s 2 2s 2 2p 6 using the notation explained below. Greg robsoncc by 20. For each electron shell atom diagram the element symbol is listed in the nucleus.
Carbon has 4 valence electrons can bond to 4 items and typically forms covalent bonds with other elements. Carbon skeletons vary in length. Concept 42 carbon atoms can form diverse molecules by bonding to four other atoms.
Carbon has 4 valence electrons can bond to 4 items and typically forms covalent bonds with other element. In writing the electron configuration for carbon the first two electrons will go in the 1s orbital. Make an electron distribution diagram of carbon.
Carbon and the molecular diversity of life. Carbon chains form skeletons. List here the types of skeletons that can be formed.
Carbon is the sixth element with a total of 6 electrons. An isotope is an alternative form of the same element containing an equal number of protons but a different number of neutrons in its atomic nucleus and thus some different properties. How many bonds can carbon form.
Fluorine greg robsoncc by 20. Neon greg robsoncc by 20. Oxygen greg robsoncc by 20.
Since 1s can only hold two electrons the next 2 electrons for c goes in the 2s orbital.
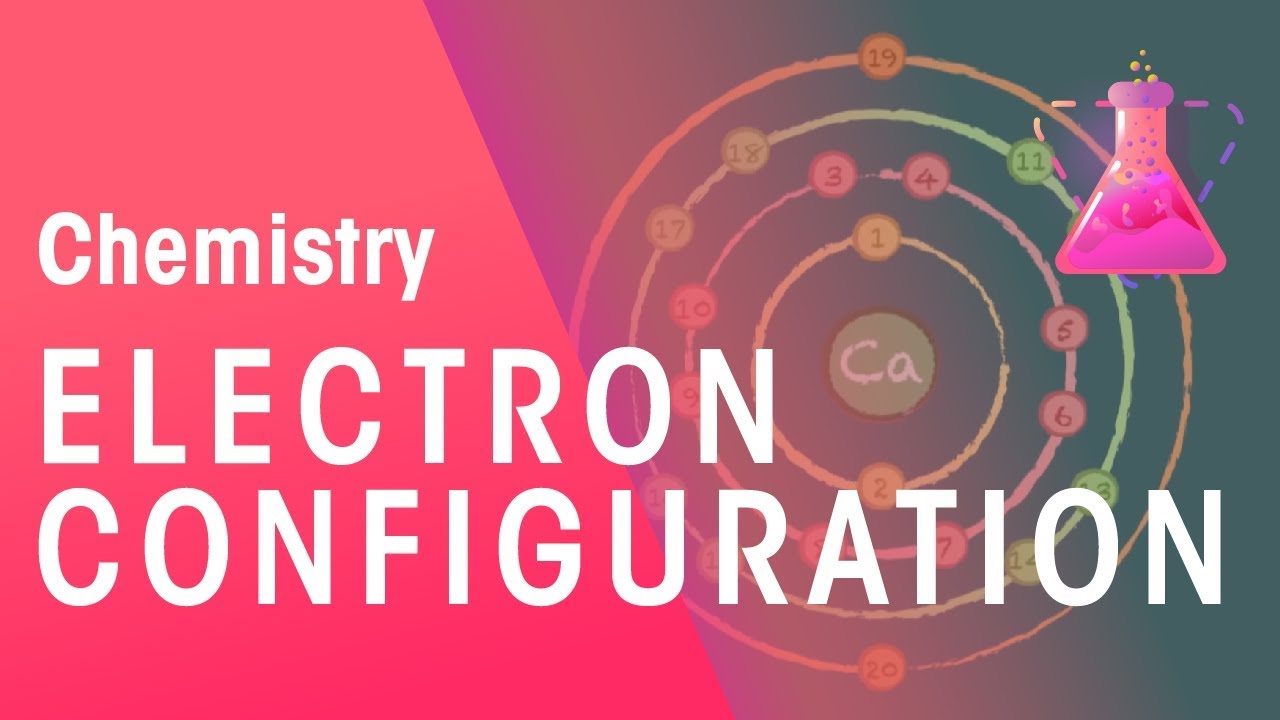 Drawing Electron Configuration Diagrams Chemistry For All
Drawing Electron Configuration Diagrams Chemistry For All
:max_bytes(150000):strip_icc()/Bromine-58b601f93df78cdcd83d2817.jpg) Atom Diagrams Electron Configurations Of The Elements
Atom Diagrams Electron Configurations Of The Elements
Electron Distribution Diagram Carbon Trusted Wiring Diagrams

:max_bytes(150000):strip_icc()/Molybdenum-58b601d73df78cdcd83d149f.jpg) Atom Diagrams Electron Configurations Of The Elements
Atom Diagrams Electron Configurations Of The Elements
:max_bytes(150000):strip_icc()/magnesiumatom-58b6026b5f9b5860464c7467.jpg) Atom Diagrams Electron Configurations Of The Elements
Atom Diagrams Electron Configurations Of The Elements
File Electron Shell 006 Carbon Svg Wikimedia Commons
 6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
 The 4 Types Of Bonds Carbon Can Form Video Lesson Transcript
The 4 Types Of Bonds Carbon Can Form Video Lesson Transcript
 Electron Configuration Boundless Chemistry
Electron Configuration Boundless Chemistry
 What Is The Molecular Orbital Energy Diagram Of Co Quora
What Is The Molecular Orbital Energy Diagram Of Co Quora
 How To Represent Electrons In An Energy Level Diagram Dummies
How To Represent Electrons In An Energy Level Diagram Dummies
 Water Electron Distribution Diagram Free Wiring Diagram For You
Water Electron Distribution Diagram Free Wiring Diagram For You
 Electron Distribution Diagram Carbon
Electron Distribution Diagram Carbon
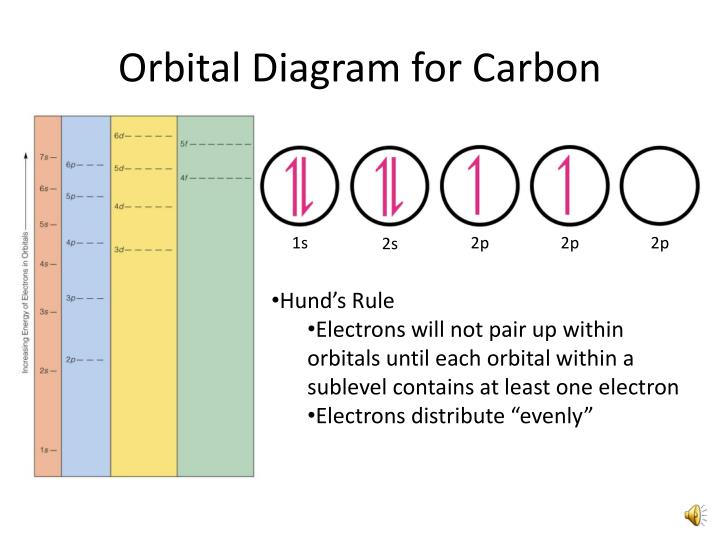 Ppt Orbital Filling Electron Configurations Powerpoint
Ppt Orbital Filling Electron Configurations Powerpoint
 Orbital Filling Electron Configurations Where Do These Electrons Go
Orbital Filling Electron Configurations Where Do These Electrons Go
 Ground State Electron Configuration Definition Example Video
Ground State Electron Configuration Definition Example Video
 How Are Electrons Distributed In Different Orbits Electronic
How Are Electrons Distributed In Different Orbits Electronic
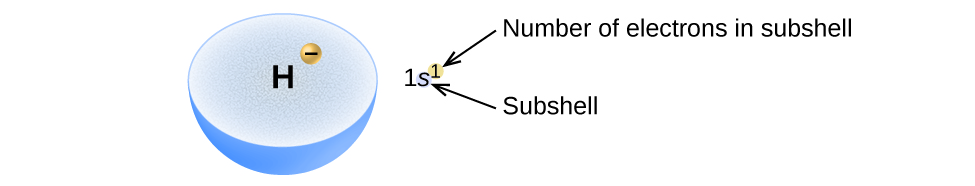 6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
 Hybridisation Mixing Up Orbitals With Sp Sp2 Sp3 Biochem Co
Hybridisation Mixing Up Orbitals With Sp Sp2 Sp3 Biochem Co
0 Response to "Electron Distribution Diagram Of Carbon"
Post a Comment