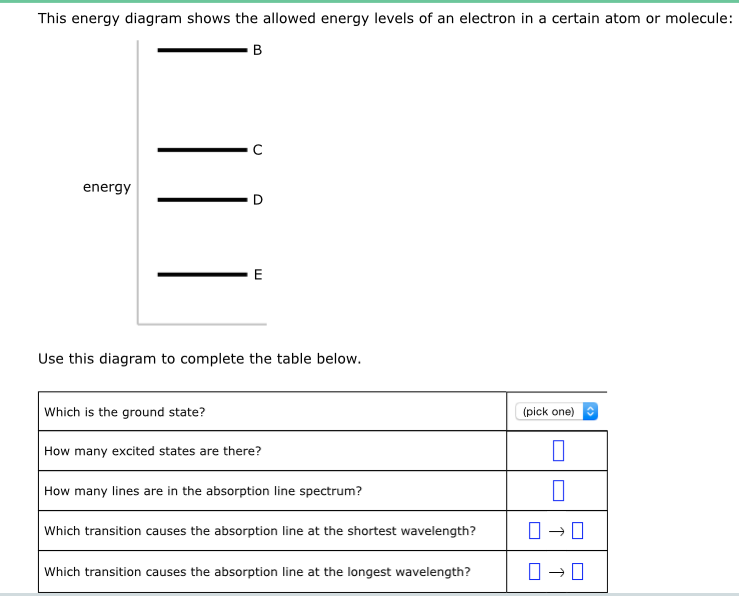
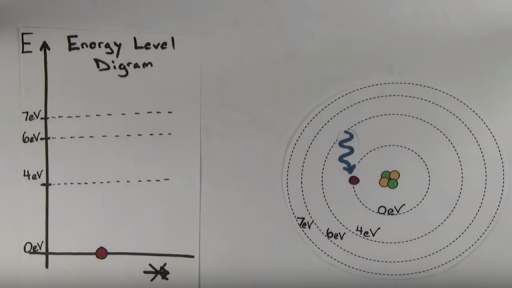
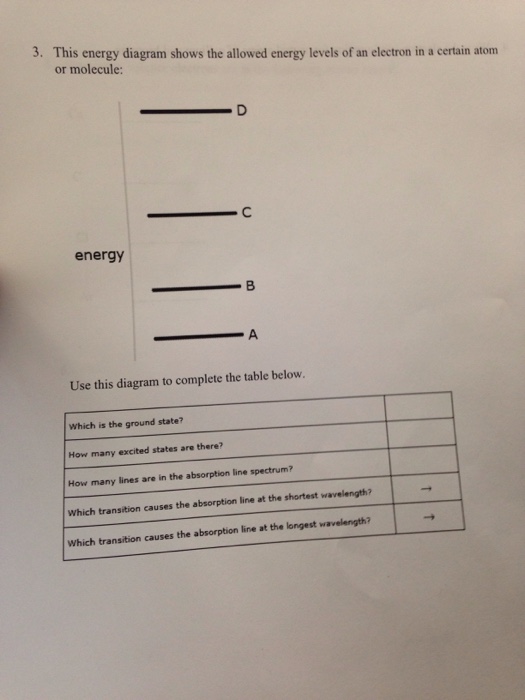
This Energy Diagram Shows The Allowed Energy Levels Of An Electron In A Certain Atom Or Molecule
To the lowest frequency light emitted. If it is in the second energy level it must have 34 ev of energy.
Figure 28 22 shows the energy levels of a certain atom.

This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule. The energy levels of an electron around a nucleus are given by. If two electrons end up in the same orbital one arrow faces up and the other faces down. Typically between 1 ev and 10 3 ev where r is the rydberg constant z is the atomic number n is the principal quantum number h is plancks constant and c is the speed of light.
Click on the image for a larger view electrons in a hydrogen atom must be in one of the allowed energy levels. So you put 8 electrons into your energy level diagram. This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule.
The photon with the highest energy is produced by the electron. Atomion with nucleus one electron. You can find the any si prefix in the aleks data tab energy z use this diagram to complete the table below.
Energy levels of electrons. The si prefix zepto means 1021. 12112016 electronic structure calculating the wavelength of a spectral line from an energy diagram this energy diagram shows the allowed energy levels of an electron in a certain atom.
You can represent electrons as arrows. Orbital state energy level. Which transition corresponds to the highest frequency light emitted.
The lowest energy state for the electrons in an atom or molecule. If an electron is in the first energy level it must have exactly 136 ev of energy. The simplest energy level diagram is that of the hydrogen atom.
Accurately predicts energy needed to remove an electron from an atom ionization. If an electron can make transitions between any two le. Shows electron transistions ie movement of electrons between energy levels.
A representation of the allowed energy states for the electrons in atoms. Allowed scientist to begin using quantum theory to explain matter at atomic level. How many spectral lines will result from all possible transition among these levels.
Use this diagram to complete the table below. The first electron goes into the 1s orbital filling the lowest energy level first and the second one spin pairs with the first one. This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule.
Consider just four of the energy levels in a certain atom as shown in shown in the diagram. This energy diagram shows the allowed energy levels of an electron in a certain atom.
 Molecules Topical Collection Bioactive Compounds
Molecules Topical Collection Bioactive Compounds
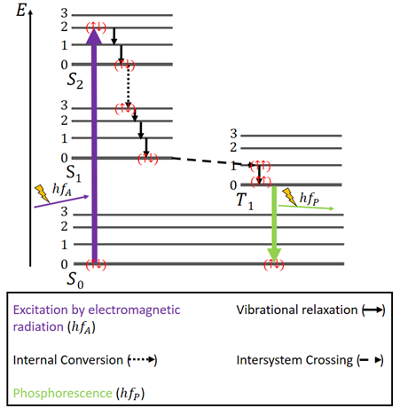 What Are Fluorescence And Phosphorescence Education
What Are Fluorescence And Phosphorescence Education
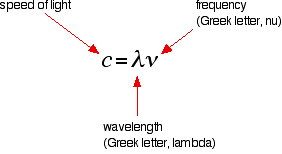 Atomic Hydrogen Emission Spectrum
Atomic Hydrogen Emission Spectrum
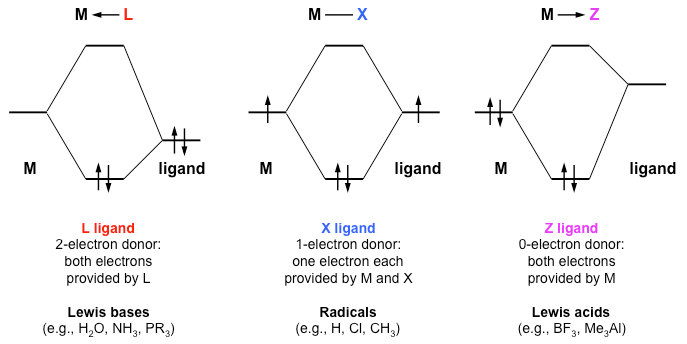 Introduction To Inorganic Chemistry Coordination Chemistry And
Introduction To Inorganic Chemistry Coordination Chemistry And
 Solved This Energy Diagram Shows The Allowed Energy Level
Solved This Energy Diagram Shows The Allowed Energy Level
 Emission And Absorption Spectra Optical Phenomena And Properties
Emission And Absorption Spectra Optical Phenomena And Properties
 Formation Of Spectral Lines Astronomy
Formation Of Spectral Lines Astronomy
Electron Configuration Boundless Chemistry
Introduction To Molecular Orbital Theory
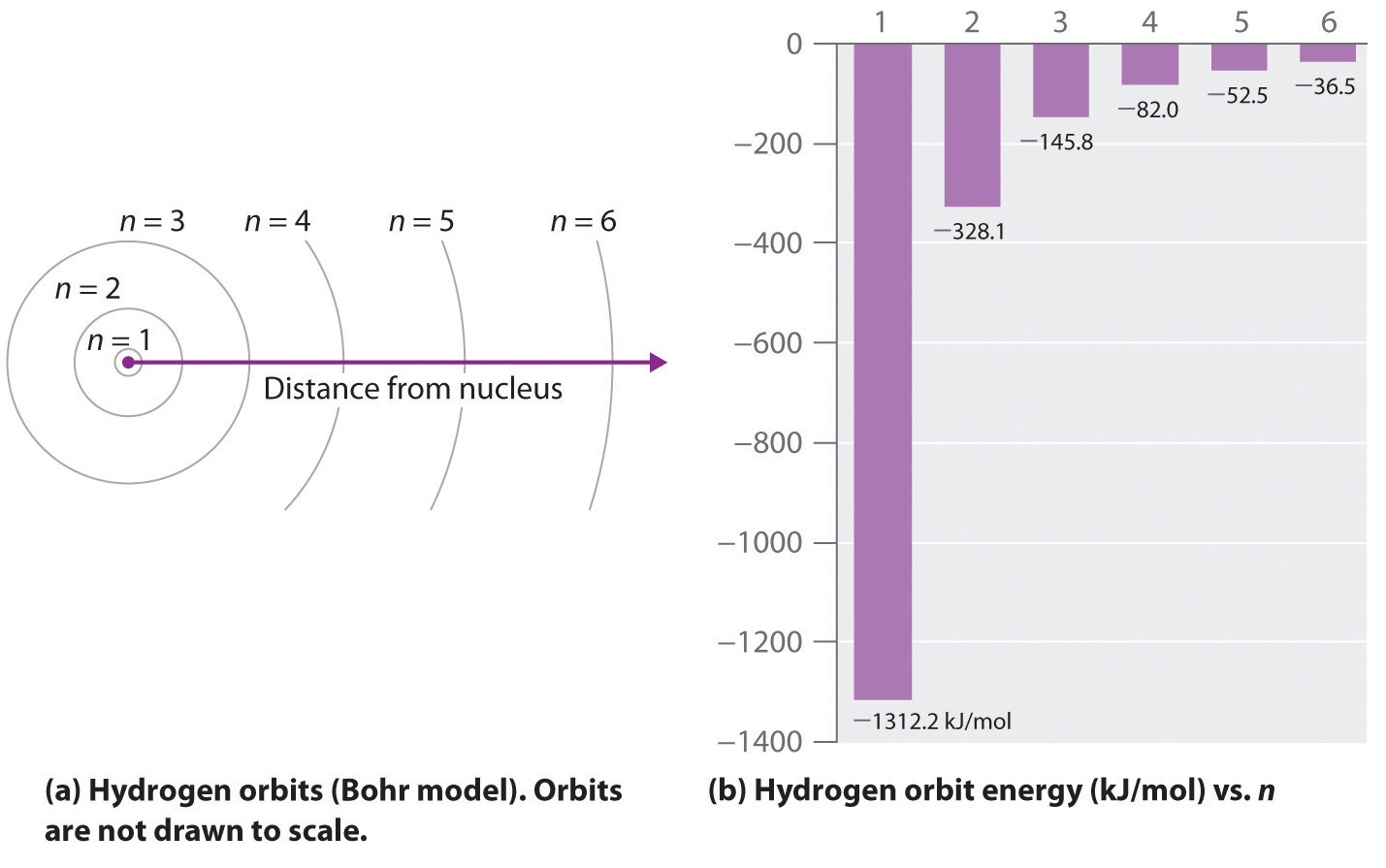 6 3 Line Spectra And The Bohr Model Chemistry Libretexts
6 3 Line Spectra And The Bohr Model Chemistry Libretexts
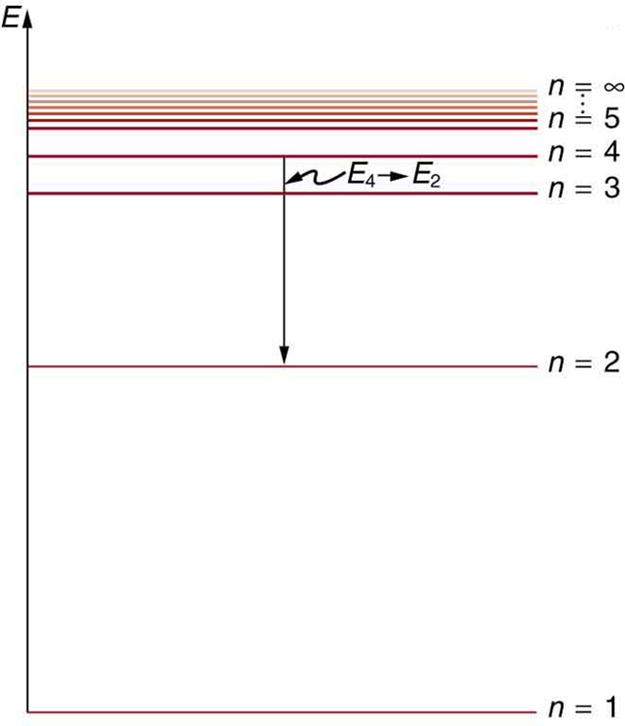 Bohr S Theory Of The Hydrogen Atom College Physics
Bohr S Theory Of The Hydrogen Atom College Physics
 Solved This Energy Diagram Shows The Allowed Energy Level
Solved This Energy Diagram Shows The Allowed Energy Level
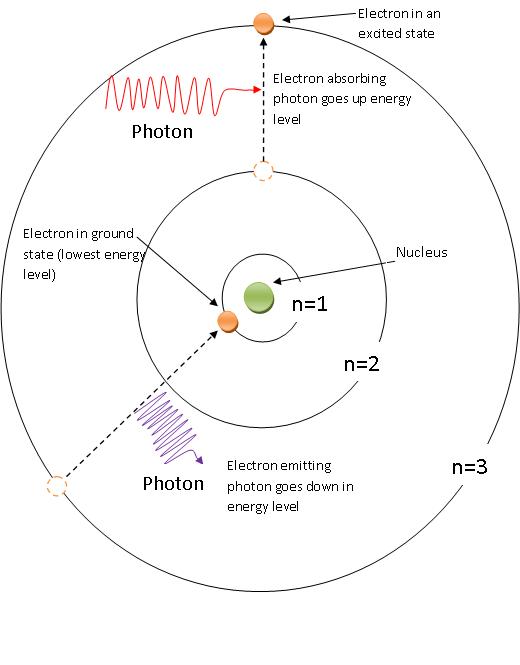 Atomic Spectra Chemistry Libretexts
Atomic Spectra Chemistry Libretexts

0 Response to "This Energy Diagram Shows The Allowed Energy Levels Of An Electron In A Certain Atom Or Molecule"
Post a Comment