What Is The Basis For Exceptions To The Aufbau Diagram
Other exceptions are copper and silver. A filled and half filled energy sublevels are more stable than partially filled energy sublevels b electron configurations are only probable.
Ch104 Chapter 2 Atoms And The Periodic Table Chemistry
Some elements have unusual atomic orbitals share to.
What is the basis for exceptions to the aufbau diagram. What is the basis for exceptions to the aufbau diagram. For example for cu with z29. Aufbau diagrams for a lithium ion.
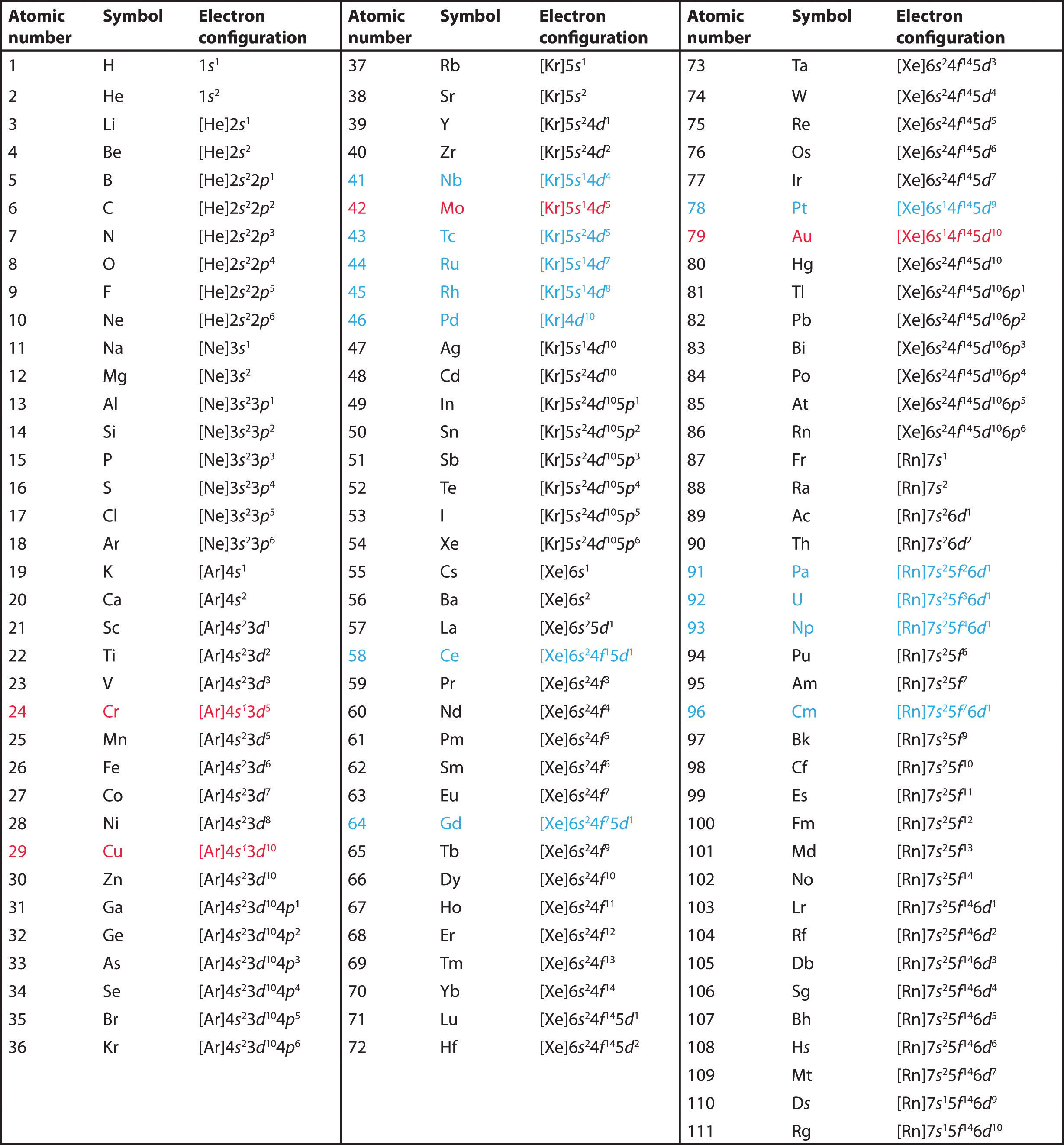
Copper and chromium are exceptions to the aufbau rule because they both have a higher spin energy than promotion energy. If we follow aufbau its condensed electronic config should be ar 4s² 3d⁹. Chemistry 1 chapter 5 test review.
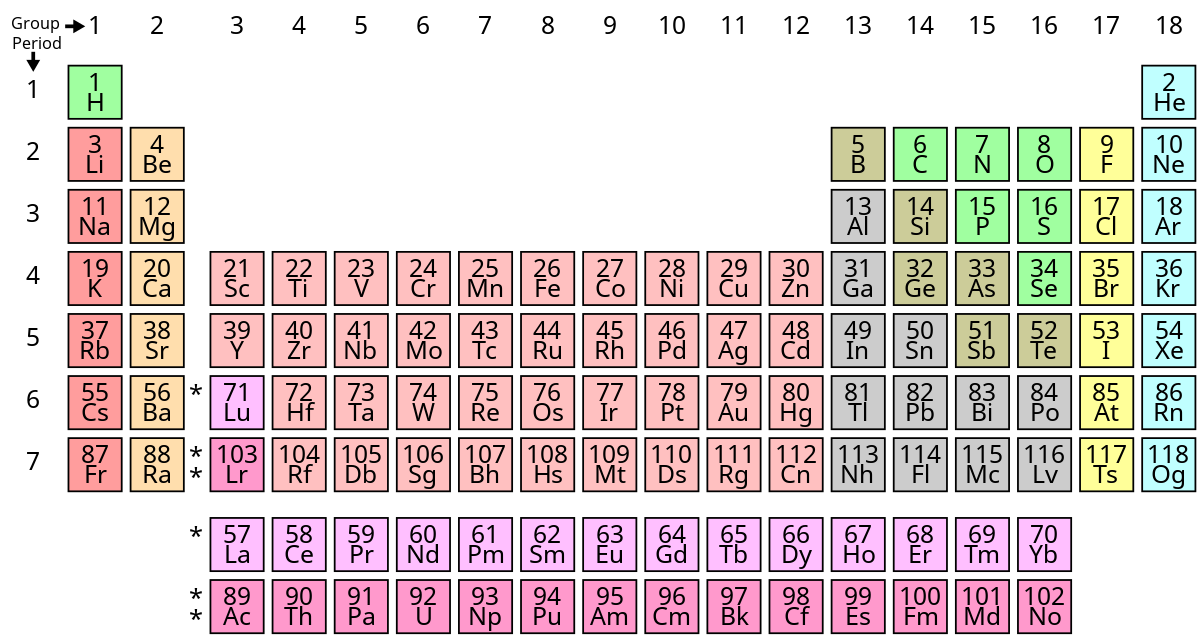
What is the basis for exceptions to the aufbau diagram. Region of high probability of finding an electron. K ca sc ti v cr mn fe co ni cu zn ga ge as se br kr.
Copper and chromium are exceptions to the aufbau rule because they both have a higher spin energy than promotion energy. What is the fourth element in the fourth period of the periodic table of elements. How does the energy of an electron change when the electron moves closer to the nucleus.
What is the basis for exceptions to the aufbau diagram. Learn vocabulary terms and more with flashcards games and other study tools. In the lower atomic numbers the difference in energy levels for the normal sequence of electron shells is larger and exceptions are not as common.
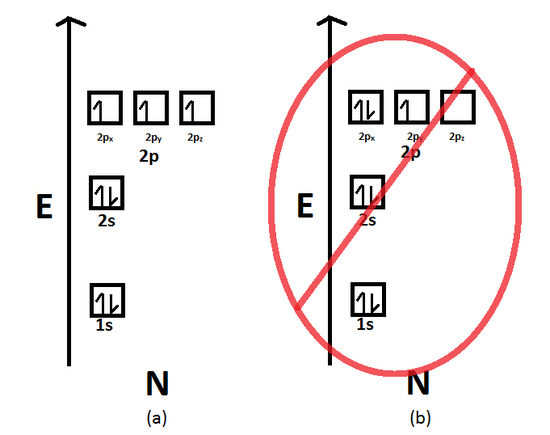
Because the promotion energy is lower than the spin pairing energy it is much easier for the electron to become promoted to the p orbital rather than spin and pair in the s orbital. Titanium the fourth period of the periodic table contain. In the first 30 elements only copper atomic number 24 and chrome atomic number 29 are exceptions to the aufbau principle.
Despite the exceptions the aufbau principle is useful in chemistry courses where students discover the fundamental rules about the atomic structure and properties of elements. But because half filled orbitals and complete orbitals are more stable an electron from the s sublevel will transfer to the d sublevel. Because the promotion energy is lower than the spin pairing energy it is much easier for the electron to become promoted to the p orbital rather than spin and pair in the s orbital.
What is the basis for exceptions to the aufbau diagrams. 1s 2s 2p 3s 3p. Start studying chemistry chapter 5.
Here the s sublevel is half filled and the d sublevel is completely filled. Filled and half filled energy sublevels are more stable than partially filled energy sublevels. Chapter 5 review electrons in atoms chapter 5 review what is the next atomic orbital in the series.
In bohrs model of the atom where are the electrons and protons located. What is the basis for exceptions to the aufbau diagram. Thus ar 4s¹ 3d¹⁰.
A chart or diagram may be used to show how the principle works for various example elements.
 Pauli Exclusion Principle An Overview Sciencedirect Topics
Pauli Exclusion Principle An Overview Sciencedirect Topics
 Electron Affinity Definition Trends Equation Video Lesson
Electron Affinity Definition Trends Equation Video Lesson
 Electron Configuration Wikipedia
Electron Configuration Wikipedia
Ch104 Chapter 2 Atoms And The Periodic Table Chemistry
 How Do Chromium And Copper Contradict The Aufbau Principle Quora
How Do Chromium And Copper Contradict The Aufbau Principle Quora
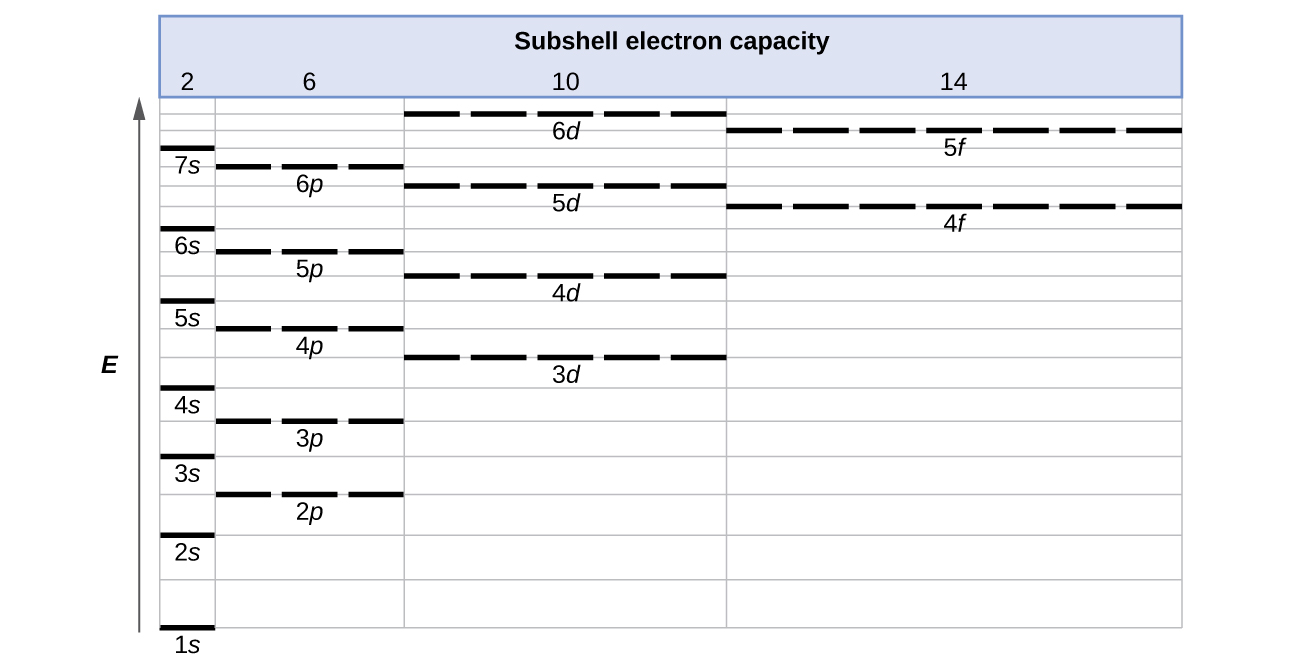 3 1 Electron Configurations Chemistry Libretexts
3 1 Electron Configurations Chemistry Libretexts
 The Fragment Molecular Orbital Method Combined With Density
The Fragment Molecular Orbital Method Combined With Density
Ch104 Chapter 2 Atoms And The Periodic Table Chemistry
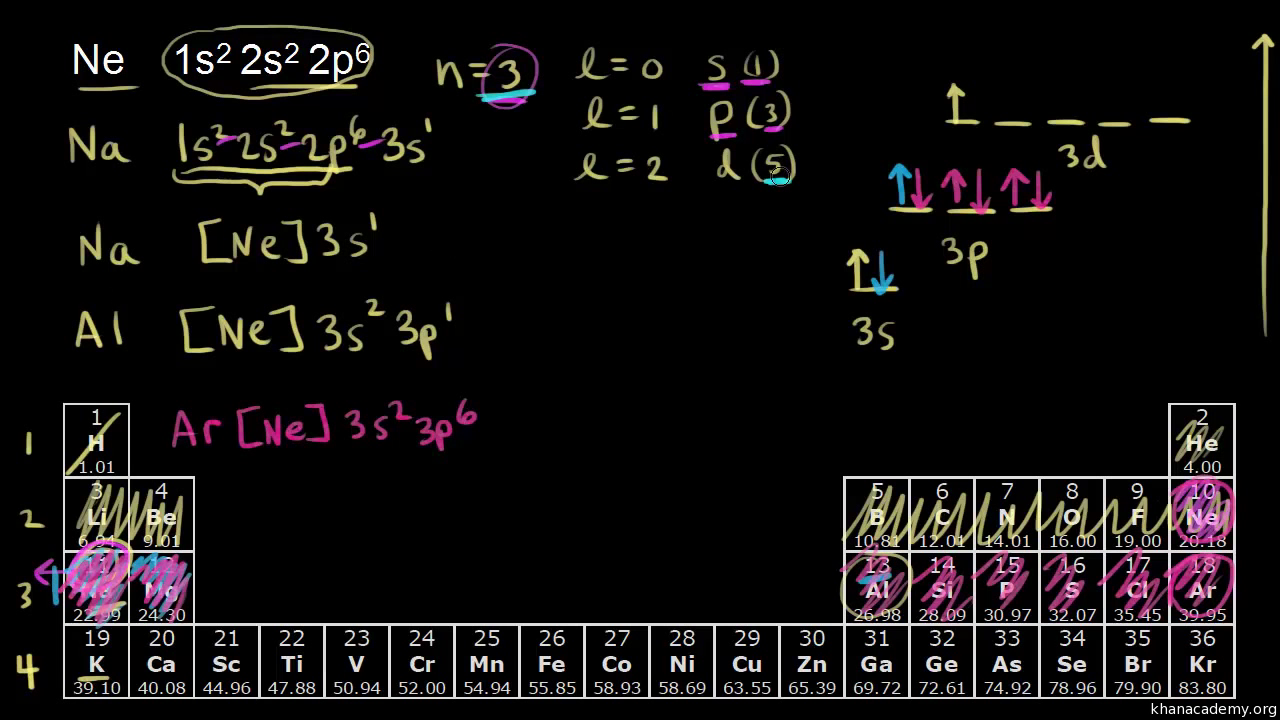 Electronic Structure Of Atoms Chemistry Science Khan Academy
Electronic Structure Of Atoms Chemistry Science Khan Academy
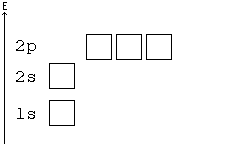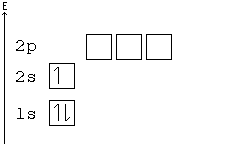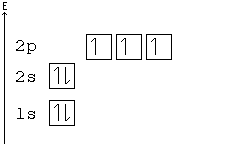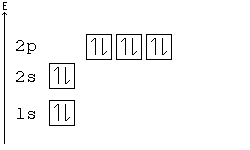 General Chemistry Filling Electron Shells Wikibooks Open Books
General Chemistry Filling Electron Shells Wikibooks Open Books
:max_bytes(150000):strip_icc()/aufbauexample-56a129555f9b58b7d0bc9f48.jpg) Madelung S Rule Definition And Example
Madelung S Rule Definition And Example
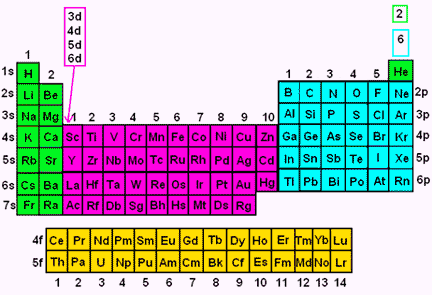 Electron Configuration Wyzant Resources
Electron Configuration Wyzant Resources
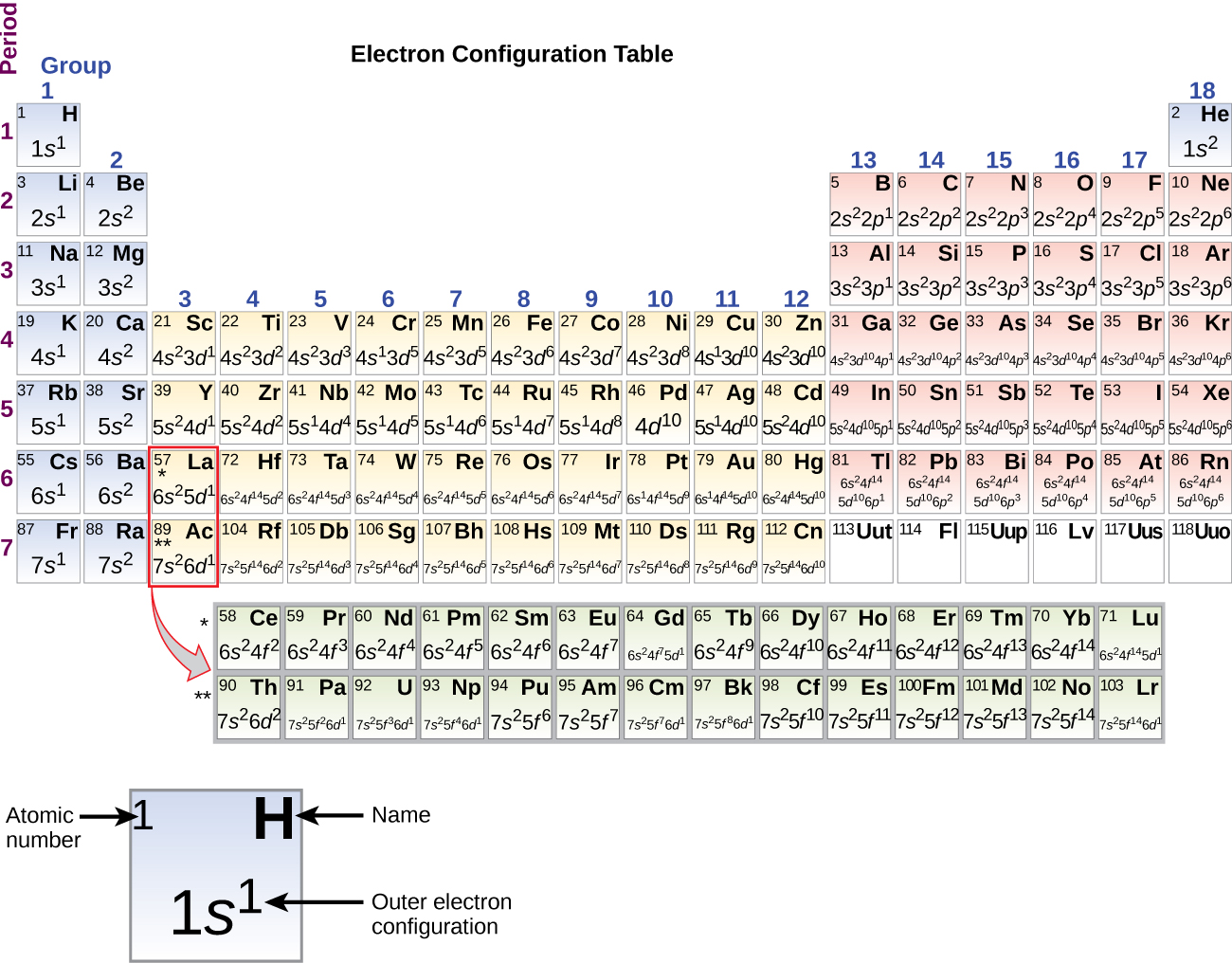 8 3 Electron Configurations How Electrons Occupy Orbitals
8 3 Electron Configurations How Electrons Occupy Orbitals
 6 9 Electron Configurations And The Periodic Table Chemistry
6 9 Electron Configurations And The Periodic Table Chemistry
 Chapter 2 6 Building Up The Periodic Table Chemistry Libretexts
Chapter 2 6 Building Up The Periodic Table Chemistry Libretexts
 Electron Configuration Boundless Chemistry
Electron Configuration Boundless Chemistry
 Electron Configuration Chemistry Libretexts
Electron Configuration Chemistry Libretexts
0 Response to "What Is The Basis For Exceptions To The Aufbau Diagram"
Post a Comment