Electron Dot Diagram For Oxygen
A step by step explanation of how to draw the lewis dot structure for o oxygen. There are 12 valence electrons available for the lewis structure for o 2.
Solved Fill In The Diagram Below For Oxygen Monofluoride
Exercises explain why the first two dots in a lewis electron dot diagram are drawn on the same side of the atomic symbol.
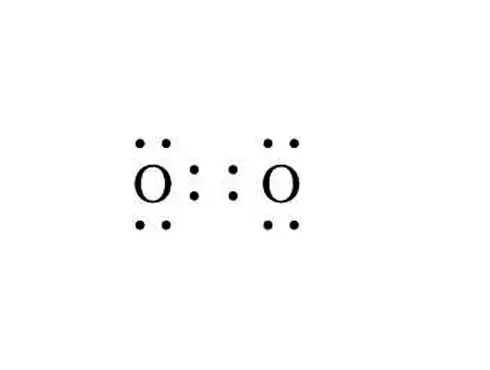
Electron dot diagram for oxygen. For diatomic nitrogen the lewis dot structure correctly predicts that there will be a triple bond between nitrogen atoms. Electron dot structure valence electrons are represented by dots placed around the chemical symbol. Lewis electron dot diagrams for ions have fewer for cations or more for anions dots than the corresponding atom.
Electrons are placed up to two on each side of the elemental symbol for a maximum of eight which is the number of electrons in a filled s and p shell. In other words if every element in group 1a has 1 valence electron then every lewis electron dot diagram would have one single dot. There are 2 bonding pairs of electrons shared between the 2 oxygen atoms and each oxygen atom also has 2 lone pairs non bonding pairs of electrons.
Generally lewis dot structures have the advantage that they are simple to work with and often present a good picture of the electronic structure. Drawing the lewis structure for o 2 dioxygen or oxygen gas oxygen o 2 is a commonly tested lewis structure due to its importance on earth. Now lets try drawing the electron dot structure of a polyatomic ion such as co 3 2.
Lewis structure electron dot diagram for the oxygen molecule o 2 or. The lewis structure for co has 10 valence electrons. The final lewis dot structure for ch 4 o would look like this.
It also is a good example of a molecule with a double bond. Carbon 4 valence e three oxygen 3 6 valence e 2 extra e to account for the negative 2 charge 24 total valence. I show you where oxygen is on the periodic table and how to determine how many valence electrons it has.
In the valence structure for the oxygen molecule each bonding pair of electrons is replaced by a dash to represent a. Lets consider another example. So the total number of valence electrons in this compound will be.
Since the lewis electron dot diagrams are based on the number of valence electrons it would hold true that the elements in the same group would have the same electron dot diagram. For the co lewis structure youll need a triple bond between the carbon and oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the co molecule.
 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
Lewis Dot Diagram For So3 Elegant Cf2s Lewis Structure How To Draw
1 2 Electron Dot Model Of Bonding Lewis Structures And Formal
Multimedia Represent Bonding With Lewis Dot Diagrams Chapter 4
 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
Diagram Of Brain Lobes Oxygen Bohr Model Michaelhannan Co
 Lewis Dot Structure For Oxygen Atom O Youtube
Lewis Dot Structure For Oxygen Atom O Youtube
 Organic Chemistry Why Can T Oxygen In Furan Be Sp Hybridized
Organic Chemistry Why Can T Oxygen In Furan Be Sp Hybridized
 Covalent Bonding With Molymods
Covalent Bonding With Molymods
Dot Diagram For Nitrogen Pretty Oxygen Molecule Diagram Oxygen Free
1 2 Electron Dot Model Of Bonding Lewis Structures And Formal
 Bohr Diagrams Compu Ibmdatamanagement Co
Bohr Diagrams Compu Ibmdatamanagement Co
Co Ordinate Dative Covalent Bonding
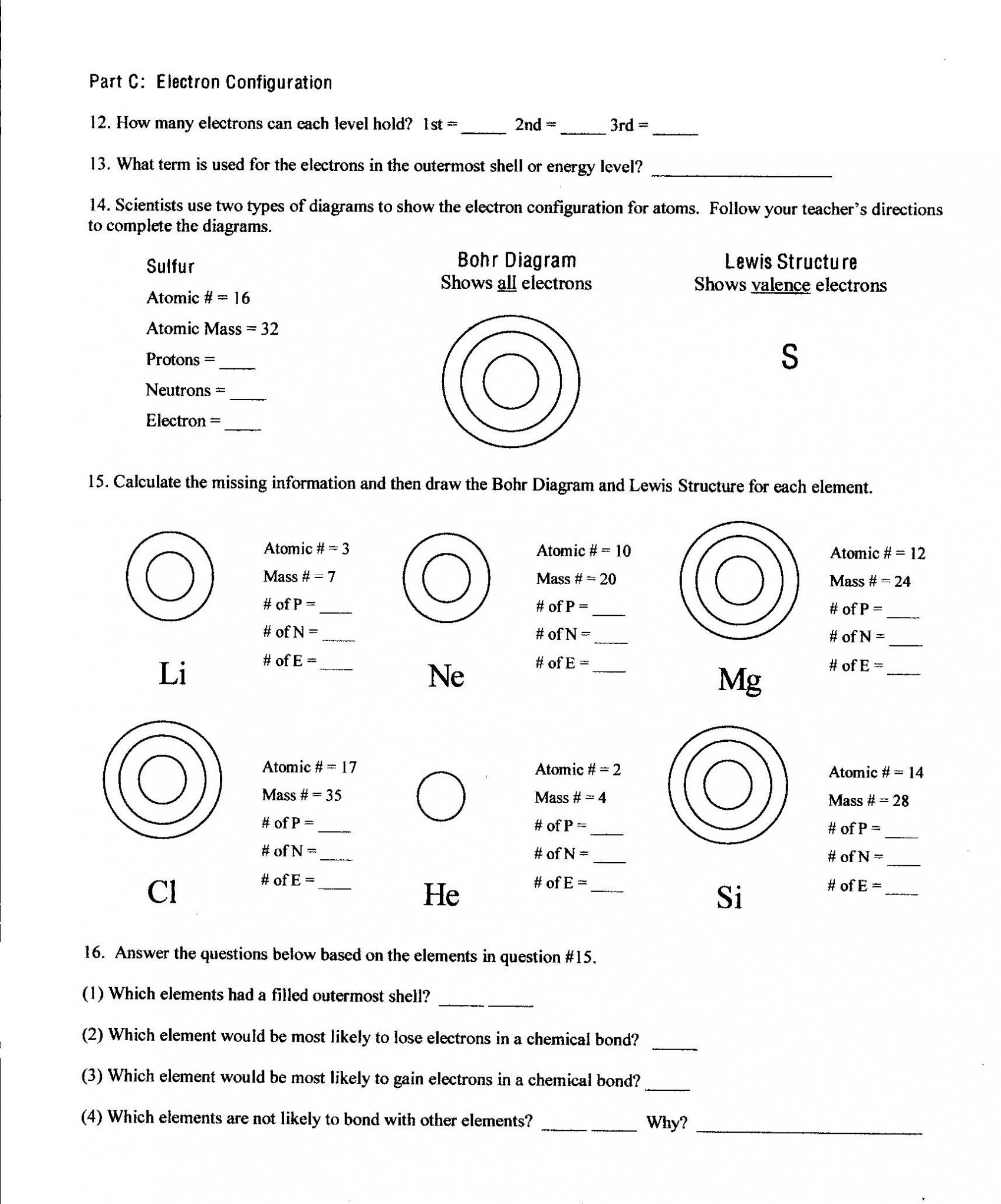 Worksheet Electron Dot Diagrams And Lewis Structures Answers
Worksheet Electron Dot Diagrams And Lewis Structures Answers
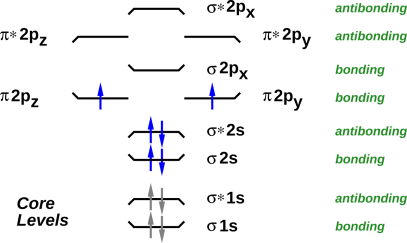 How To Draw Molecules And Chemical Bonds Just Like Lewis Dot
How To Draw Molecules And Chemical Bonds Just Like Lewis Dot
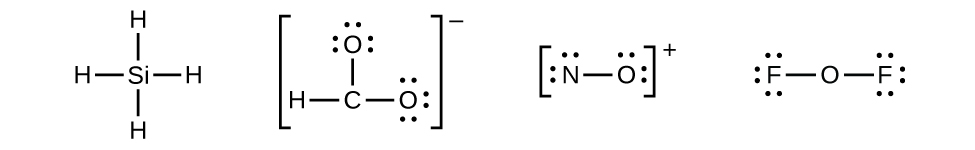 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
 Lewis Dot Diagram For Oxygen Elegant Lewis Structure Example Problem
Lewis Dot Diagram For Oxygen Elegant Lewis Structure Example Problem
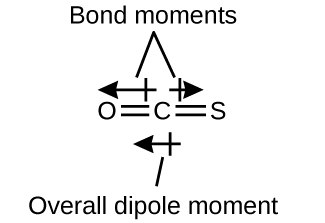 7 6 Molecular Structure And Polarity Chemistry
7 6 Molecular Structure And Polarity Chemistry
1 2 Electron Dot Model Of Bonding Lewis Structures And Formal
Co2 Dot Diagram Michaelhannan Co
Oxygen Free Radical Electron Dot Diagram
 How To Determine The Lewis Dot Structure Of O2 Quora
How To Determine The Lewis Dot Structure Of O2 Quora

0 Response to "Electron Dot Diagram For Oxygen"
Post a Comment