Use The Orbital Diagram For Oxygen To Write Quantum Numbers For The 4th Electron Of The O Atom
Write the electron configuration for phosphorus and draw the orbital diagram. How to write the electron configuration for oxygen.
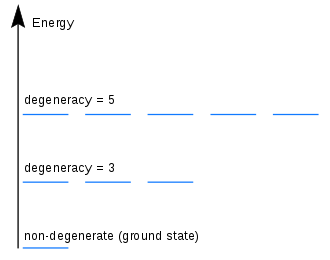 Degenerate Energy Levels Wikipedia
Degenerate Energy Levels Wikipedia
N represents the energy level l is associated with the sublevel ml represents the orbital and ms is the electron spin.

Use the orbital diagram for oxygen to write quantum numbers for the 4th electron of the o atom. The remaining four electrons will go in the 2p orbital. The orbital filling diagram of lithium. If two electrons end up in the same orbital one arrow faces up and the other faces down.
So you put 8 electrons into your energy level diagram. Specifies the energy of an electron and the size of the orbital the distance from the nucleus of the peak in a radial probability distribution plot. It discusses the 4 quantum numbers n l ml and ms.
The electron configuration for phosphorus. You can represent electrons as arrows. Orbitals are built up from atom to atom.
Since 1s can only hold two electrons the next 2 electrons for o go in the 2s orbital. This number means that oxygen has 8 protons in its nucleus and 8 electrons. The first three quantum numbers of an electron are n1.
In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. When writing the electron configuration for an atom orbitals are filled in order of increasing atomic number. All orbitals that have the same value of n are said to be in the same shell level.
The pauli exclusion principle states that no two electrons in an atom can have the same four quantum numbers electrons in the same orbital must. N 1 2 3. The diagram shows the number of subshell by using boxes or lines for electrons use three for p orbitals five for d orbitals and 7 for f orbitals.
In order to write the o electron configuration we first need to know the number of electrons for the o atom. This means that there are two electrons in the 1s orbital and one electron in the higher energy 2s orbital. The electron configuration of lithium is 1s²2s¹.
Electron configuration and orbital diagrams part 1. Oxygen is the eighth element with a total of 8 electrons. For news about the other quantum numbers you really should check the appropriate tutorial.
1 charge means lost one electron write the normal electron configuration for na then remove an electron from the last orbital. In addition to listing the principle quantum number n and the subshell ell the orbital diagram shows all the different orientations and the spin of every electron. A step by step description of how to write the electron configuration for oxygen o.
Principal quantum number n.
 Electron Configurations The Periodic Table
Electron Configurations The Periodic Table
 6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
 Magnetic Quantum Number Definition Example Video Lesson
Magnetic Quantum Number Definition Example Video Lesson
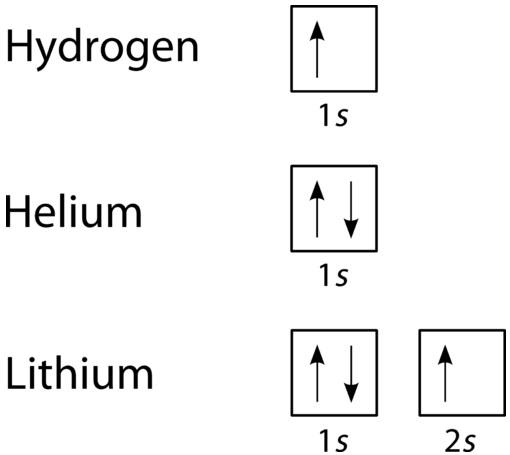 9 6 Quantum Mechanical Orbitals And Electron Configurations
9 6 Quantum Mechanical Orbitals And Electron Configurations
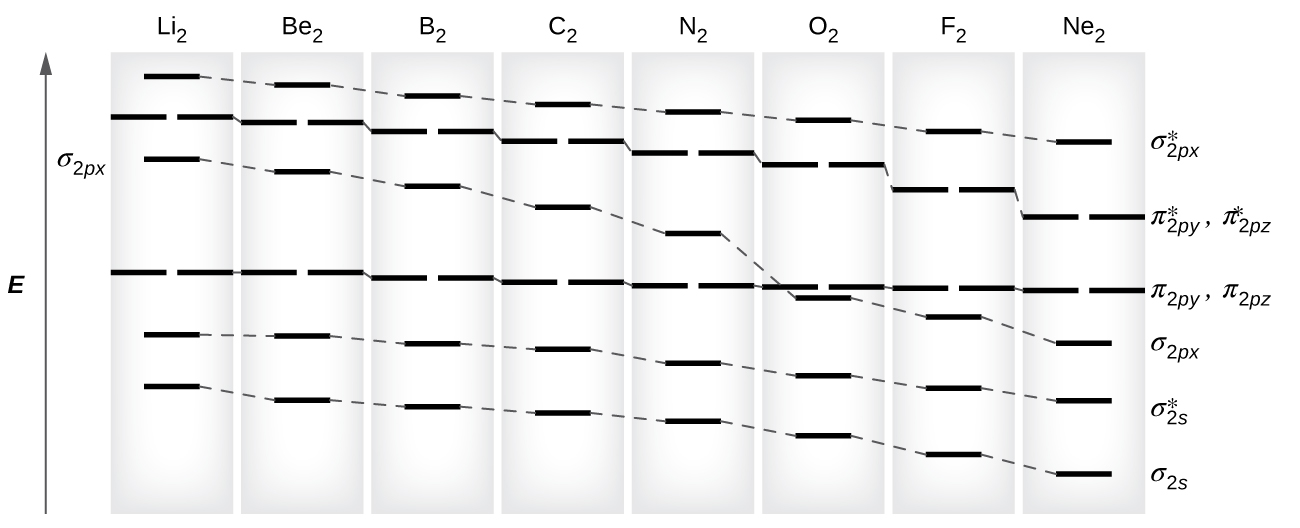 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
Chem4kids Com Oxygen Orbital And Bonding Info
 High School Chemistry Electron Configurations Of Main Group Elements
High School Chemistry Electron Configurations Of Main Group Elements
 Electronic Configurations Using Arrows Youtube
Electronic Configurations Using Arrows Youtube
 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
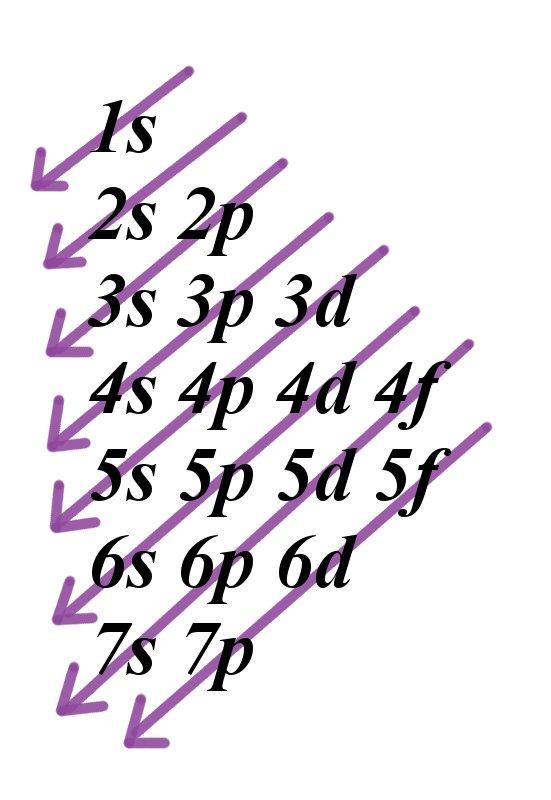 Electronic Configurations Intro Chemistry Libretexts
Electronic Configurations Intro Chemistry Libretexts
 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
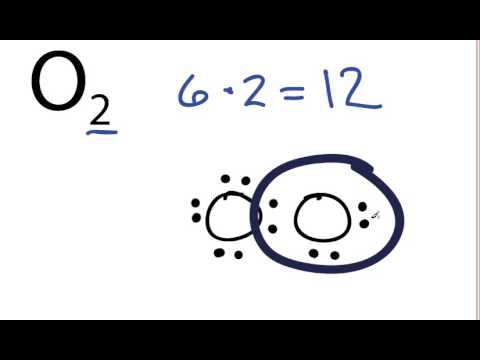 O2 Lewis Structure How To Draw The Lewis Structure For Oxygen Gas
O2 Lewis Structure How To Draw The Lewis Structure For Oxygen Gas
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
Chem4kids Com Oxygen Orbital And Bonding Info

 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 What Is The Correct Set Of Quantum Numbers N L Ml Ms For The
What Is The Correct Set Of Quantum Numbers N L Ml Ms For The
0 Response to "Use The Orbital Diagram For Oxygen To Write Quantum Numbers For The 4th Electron Of The O Atom"
Post a Comment