Study The Following Reaction Energy Diagram
Rates and mechanisms of chemical reactions. Home study science chemistry chemistry questions and answers consider the following reaction energy diagram.
 Le Chatelier S Principle Chemical Equilibrium Siyavula
Le Chatelier S Principle Chemical Equilibrium Siyavula
Ea forward ea reverse if deltah0 which of the following statements correctly describe the key aspects of drawing a reaction energy diagram.

Study the following reaction energy diagram. Compare the amounts of reactants and products that will be present when the system reaches equilibrium. Quiz worksheet energy reaction coordinate. View the full answer.
Sketch the reaction energy diagram for this reaction reactants on the left products on the right and transition state in the middle. Part a the reactants are at a lower energy than the products. Based on the following energy diagram the heat of reaction for the reaction represented is approximately 20 kcal.
2 hbrg cl2g 2 hclg br2gk 0. What is a transition state. Use the following enthalpy diagram to answer the following questions.
An isolable intermediate in a reaction. A local maximum on the energy diagram. Sketch the reaction energy diagram for this reaction reactants on the left products on the right and transition state in the middle.
Endothermic the products are at a lower energy level. The above reaction is a propagation reaction as we see a radicalch3 in the reactant has reacted to form a free radical in the productcl. How many elementary steps are in the reaction me.
Sketch the reaction energy diagram for this reaction onsider the following reaction and its value of k. Therefore energy is absorbed. Consider the following reaction energy diagram.
How many elementary steps are in the reaction. Which of the following energy diagrams shows the reaction with the smallest energy of activation. Reaction energy diagram is the graph of reaction progress versus energy.
Part b the transition states in the reaction correspond. Draw an energy diagram for a concerted ie. The vertical axis represents the overall energy of the reactants and the horizontal axis represents the reaction progress.
For an exothermic reaction the energy of the products is less than the energy of the reactants. Use the following enthalpy diagram to answer the following questions. Which point on the following reaction coordinate diagram represents the activation energy.
Refer to the lesson titled analyzing energy with a. The starting materials of the reaction. This indicates that the reactants have absorbed energy to form the products.
Studying Chemical Reactions With Gaussian
 Chemical Engineering Software Model Chemical Units And Reactors
Chemical Engineering Software Model Chemical Units And Reactors
 Dft Study Of The Orr On Au Surfaces In Alkaline Solutions A D
Dft Study Of The Orr On Au Surfaces In Alkaline Solutions A D
 How To Draw Label Enthalpy Diagrams Study Com
How To Draw Label Enthalpy Diagrams Study Com
Reaction Rates Chemical Kinetics

Reaction Rates Chemical Kinetics
 Chem2710 Exam 2 Study Guide 17 1 Doc Chemistry 2710 Exam 2 Study
Chem2710 Exam 2 Study Guide 17 1 Doc Chemistry 2710 Exam 2 Study
 Le Chatelier S Principle Chemical Equilibrium Siyavula
Le Chatelier S Principle Chemical Equilibrium Siyavula
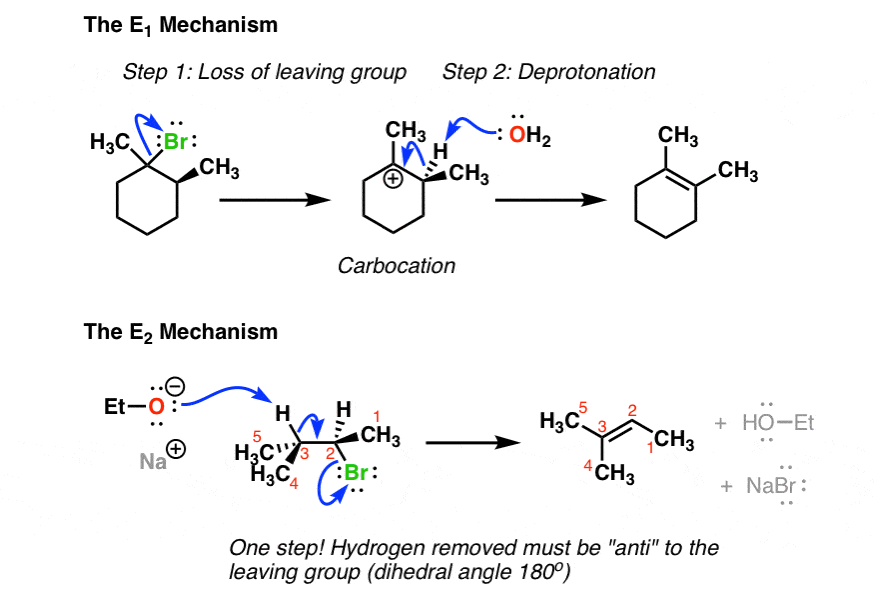 Comparing The E1 And E2 Reactions Master Organic Chemistry
Comparing The E1 And E2 Reactions Master Organic Chemistry
 Activation Energy An Overview Sciencedirect Topics
Activation Energy An Overview Sciencedirect Topics
 Mcat General Chemistry Review Summary Gold Standard Mcat Prep
Mcat General Chemistry Review Summary Gold Standard Mcat Prep
Energy Diagram Module Series Part Three Intermediates And Rate
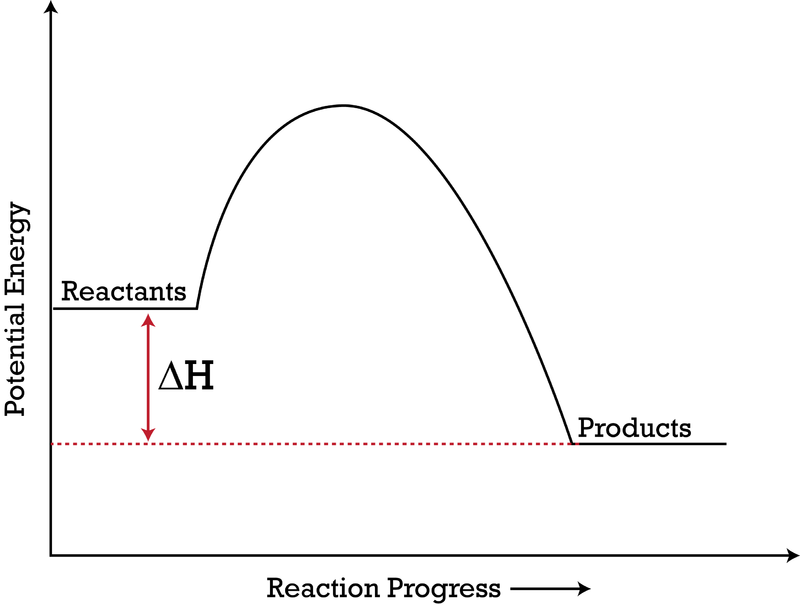
 Ppt Reaction Rates 2 Powerpoint Presentation Id 3493687
Ppt Reaction Rates 2 Powerpoint Presentation Id 3493687
 Photosynthesis Importance Process Reactions Britannica Com
Photosynthesis Importance Process Reactions Britannica Com
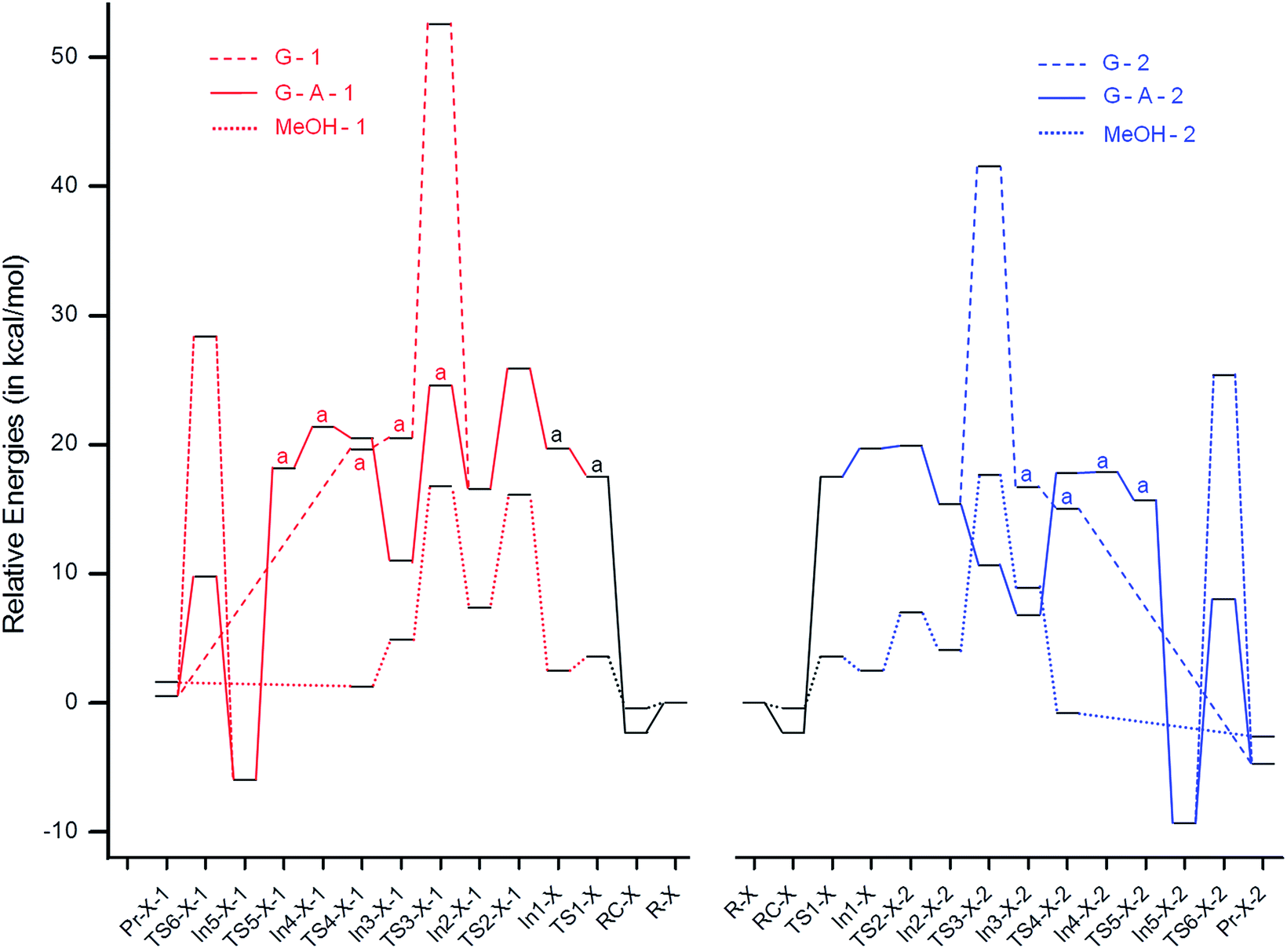 Insights Into The Morita Baylis Hillman Reaction Of Isomeric
Insights Into The Morita Baylis Hillman Reaction Of Isomeric
 2 2b Reaction Rates Chemical Equilibrium Interpret Diagram
2 2b Reaction Rates Chemical Equilibrium Interpret Diagram
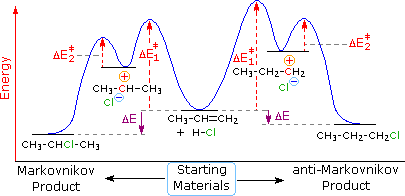

0 Response to "Study The Following Reaction Energy Diagram"
Post a Comment