Molecular Orbital Diagram Khan Academy
Watch these lectures to gain a better understanding of orbitals and the structure of an atom. But just to review the s orbital makes a circle or sphere.
 Chemistry Molecular Orbital Diagrams
Chemistry Molecular Orbital Diagrams
Orbitals are what happen.
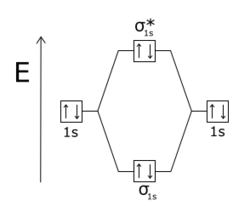
Molecular orbital diagram khan academy. This is a very basic introduction to molecular orbital theory. Jpg when two h atoms get close enough their orbitals merge to include both nuclei. Hybrid orbitals and molecular orbital theory duration.
Between molecules like n2 o2 and others like hf. Lewis vsepr valence orbitals and mo. Introduction to molecular orbital theory.
Just so you get a little bit more notation so when people talk about hybridized sp3 orbitals all theyre saying is look carbon doesnt bond. Using a wealth of examples to depict molecular orbitals mos formed by the linear combination of atomic orbitals lcao. The simplest atomic orbital is the spherical 1s orbital of hydrogen.
We then have a molecular orbital. Pi bonds and sp2 hybridized orbitals structure and bonding organic chemistry khan academy. Professor sylvia ceyer covers the molecular orbital theory beginning with a discussion of some key topics including bonding orbitals antibonding orbitals electron configurations and bond order.
Molecular orbital theory bonding antibonding mo bond order homonuclear diatomic molecules. It covers the basics of how to solve for bond order. A molecular orbital extends over more than one atom.
The d orbital makes a double dumbbell or crisscross shape in the x y or z plane. You were introduced to the shapes the orbitals make in another video. The p orbital makes a dumbbell shape in either the x y or z plane.
The periodic table of the elements log in or sign up to track your course progress gain access to final exams and get a free certificate of completion. Bonding and anti bonding by yifeng zhu. Orbitals and electron configurations atoms have a specific structure that determines their behavior in an element or compound.
So this is how the hydrogen orbital and the carbon orbitals get mixed. You wont necessarily find it there 100 of the time. The hydrogens 1s orbital bonds with well each of the hydrogens 1s orbital bonds with each of the carbons sp3 orbitals.
In mo theory explaining bonding anti bonding and non bonding orbitals in general and how to fill the electrons in the orbitals. Bonding and antibonding molecular orbitals. An orbital is a region in space where an electron is most likely to be found.
Orbitals and electron configuration back to 24. And orbitals are more like probability functions as to where you might find the electron while an orbit is a very kind of classical mechanical way of describing the path of a classical object like a planet around a star.
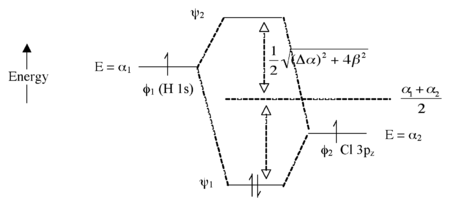 Introduction To Inorganic Chemistry Molecular Orbital Theory
Introduction To Inorganic Chemistry Molecular Orbital Theory
 What Is The Molecular Orbital Energy Diagram Of Co Quora
What Is The Molecular Orbital Energy Diagram Of Co Quora
 Why Molecular Orbital Diagram Of Oxygen Is Diffrent From Nitrogen
Why Molecular Orbital Diagram Of Oxygen Is Diffrent From Nitrogen
 Construct The Orbital Diagram For F Sapling Schematic Diagram
Construct The Orbital Diagram For F Sapling Schematic Diagram
 Molecular Orbitals And Hybridizations Organic Chemistry Socratic
Molecular Orbitals And Hybridizations Organic Chemistry Socratic
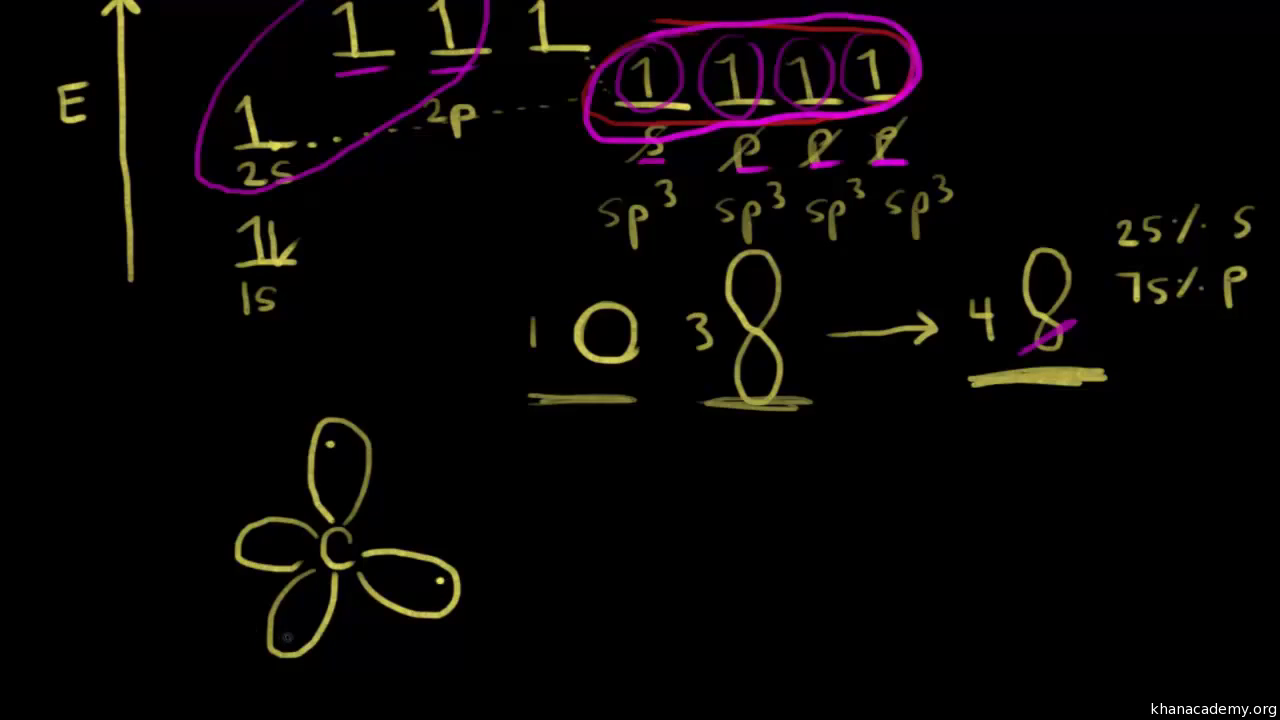 Sp Hybridization Hybrid Orbitals Chemical Bonds Video Khan
Sp Hybridization Hybrid Orbitals Chemical Bonds Video Khan
 Antibonding Molecular Orbital Wikipedia
Antibonding Molecular Orbital Wikipedia
Molecular Orbitals Introductory Chemistry 1st Canadian Edition
 What Is The Molecular Orbital Energy Diagram Of Co Quora
What Is The Molecular Orbital Energy Diagram Of Co Quora
 Molecular Orbital Diagram For B2 Luxury C2 N2 Molecular Orbital
Molecular Orbital Diagram For B2 Luxury C2 N2 Molecular Orbital
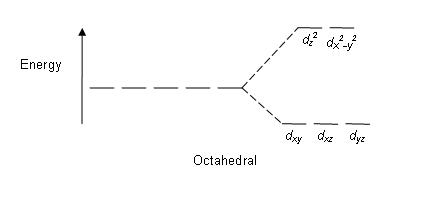 Crystal Field Theory Wikipedia
Crystal Field Theory Wikipedia
 P Orbital Diagram Delocalized Bonding And Molecular Orbitals
P Orbital Diagram Delocalized Bonding And Molecular Orbitals
 Molecular Orbital Diagram For B2 Luxury C2 N2 Molecular Orbital
Molecular Orbital Diagram For B2 Luxury C2 N2 Molecular Orbital
 Draw The Molecular Orbital Diagram For I Be2 Ii B2 And Predict
Draw The Molecular Orbital Diagram For I Be2 Ii B2 And Predict
Molecular Orbitals Introductory Chemistry 1st Canadian Edition
How To Draw Molecular Orbital Diagram H2 Mo Diagram Daytonva150
 Chapter 9 Covalent Bonding Orbitals Copyright C Cengage Learning
Chapter 9 Covalent Bonding Orbitals Copyright C Cengage Learning
 Bonding And Antibonding Orbitals Chemistry Libretexts
Bonding And Antibonding Orbitals Chemistry Libretexts
 3 Ways To Calculate Bond Order In Chemistry Wikihow
3 Ways To Calculate Bond Order In Chemistry Wikihow
 Introduction To Inorganic Chemistry Molecular Orbital Theory
Introduction To Inorganic Chemistry Molecular Orbital Theory
0 Response to "Molecular Orbital Diagram Khan Academy"
Post a Comment