O2 Molecular Orbital Diagram
From the molecular orbital diagram we observe that oxygen has two unpaired electrons which is consist with the paramagnetic nature of oxygen. These mo diagrams can explain why some molecules exist and others do not.
Mo Diagrams For Diatomic Molecules
The oxygen atomic orbitals are labeled according to their symmetry as a 1 for the 2s orbital and b 1 2p x b 2 2p y and a 1 2p z for the three 2p orbitals.

O2 molecular orbital diagram. In o 2 and f 2 there is a crossover of the sigma and the pi ortbials. The molecular orbital diagram shown below can be used to describe the bonding in oxygen o 2 fluorine f 2 non ne 2 and hypofluorite of. The relative energies of the sigma orbitals drop below that of the pi orbitals.
Bonding order is 2 and it is paramagnetic. From that diagram you can then easily fill out what the o2 and o2 mo diagrams should beand that is in the second photo i included. The two hydrogen 1s orbitals are premixed to form a 1 σ and b 2 σ mo.
This video shows the construction of a molecular orbital mo diagram for the diatomic molecule o2 using the valence electrons of each oxygen. Fill from the bottom up with 12 electrons total. Molecular orbitals mo are constructed from atomic orbitals.
As you know oxygen is located in period 2 group 16 of the periodic table and has an atomic number equal to 8. Water h 2o is a bent molecule 105 with c 2v molecular symmetry. The only orbitals that are important in our discussion of molecular orbitals are those formed when valence shell orbitals are combined.
Increase the fertility of soil with prime laboratories providing the best quality hydrogel for agriculture acting as a controlled water release system by releasing water only upon plant requirement. In order to draw oxygens molecular orbital diagram you need to start by taking a look at what atomic orbitals you have for an oxygen atom o. The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals.
The o2 mo diagram will have one more electron compared to o2 and the o2 mo diagram will have one fewer electron compared to o2. Molecular orbital diagram for oxygen gas o2. Information from the mo diagram justify o2s stability and show that its bonding order is 2.
The orbitals are arranged from the lowest energy molecular orbital to the highest molecular orbital. In the o2 molecular orbital diagram when creating the molecular orbitals from the p orbitals notice the three atomic orbitals split into three molecular orbitals a singly degenerate σ and a doubly degenerate π orbital.
 Molecular Orbital Mo Diagram Of O2 Youtube
Molecular Orbital Mo Diagram Of O2 Youtube
Chem 344 Homework 9 Due Friday Apr 11 2014 2 Pm
What Is The Molecular Orbital Diagram For O2 And O2 Ions Quora
 Constructing The O2 Molecular Orbital Energy Level Diagram Youtube
Constructing The O2 Molecular Orbital Energy Level Diagram Youtube
 Energy Level Diagram For Molecular Orbitals Chemical Bonding And
Energy Level Diagram For Molecular Orbitals Chemical Bonding And
Unit I Molecular Structure And Theories Of Bonding Terms And
 On The Properties Of O And O2 Ions In A Hybrid Model And In Mars
On The Properties Of O And O2 Ions In A Hybrid Model And In Mars
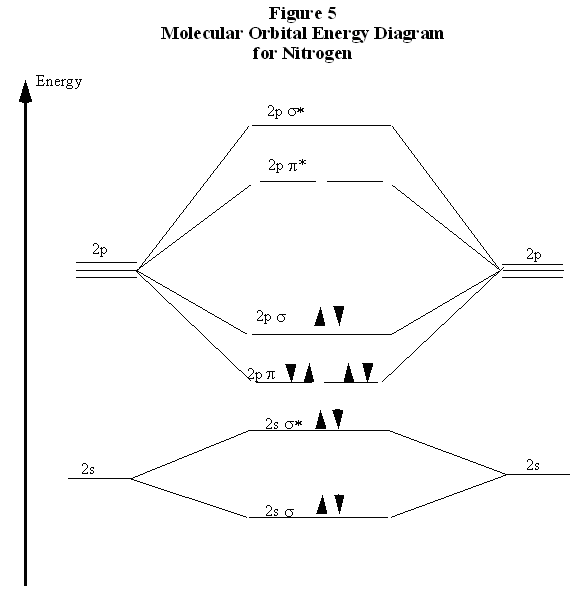 Ionization Energies Of Diatomic Molecule Chemistry Libretexts
Ionization Energies Of Diatomic Molecule Chemistry Libretexts
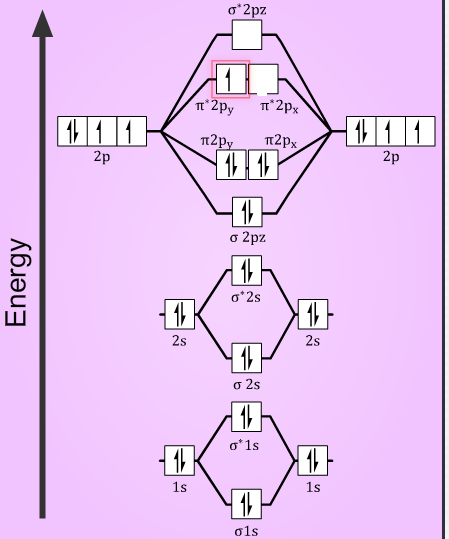 More Stable Among O2 And O2 Chemical Bonding And Molecular
More Stable Among O2 And O2 Chemical Bonding And Molecular
 On Average How Many Pi Bonds Does O 2 Have Socratic
On Average How Many Pi Bonds Does O 2 Have Socratic
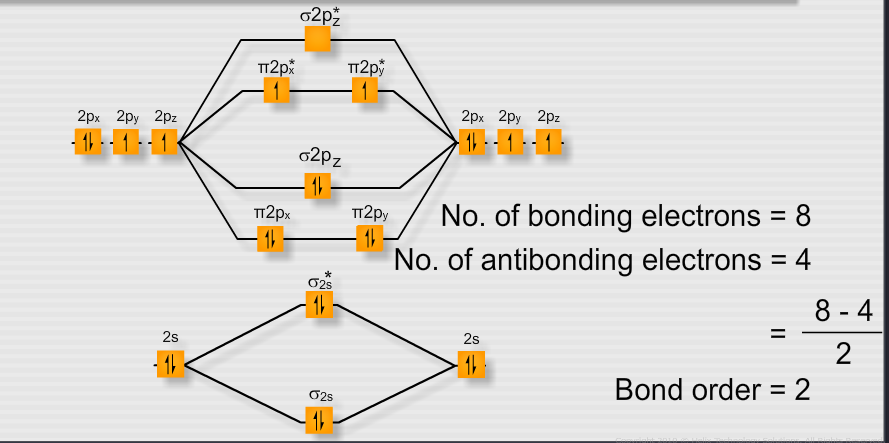 Which Has More Bond Dissociation Energy Why O2 Or O2 Chemical
Which Has More Bond Dissociation Energy Why O2 Or O2 Chemical
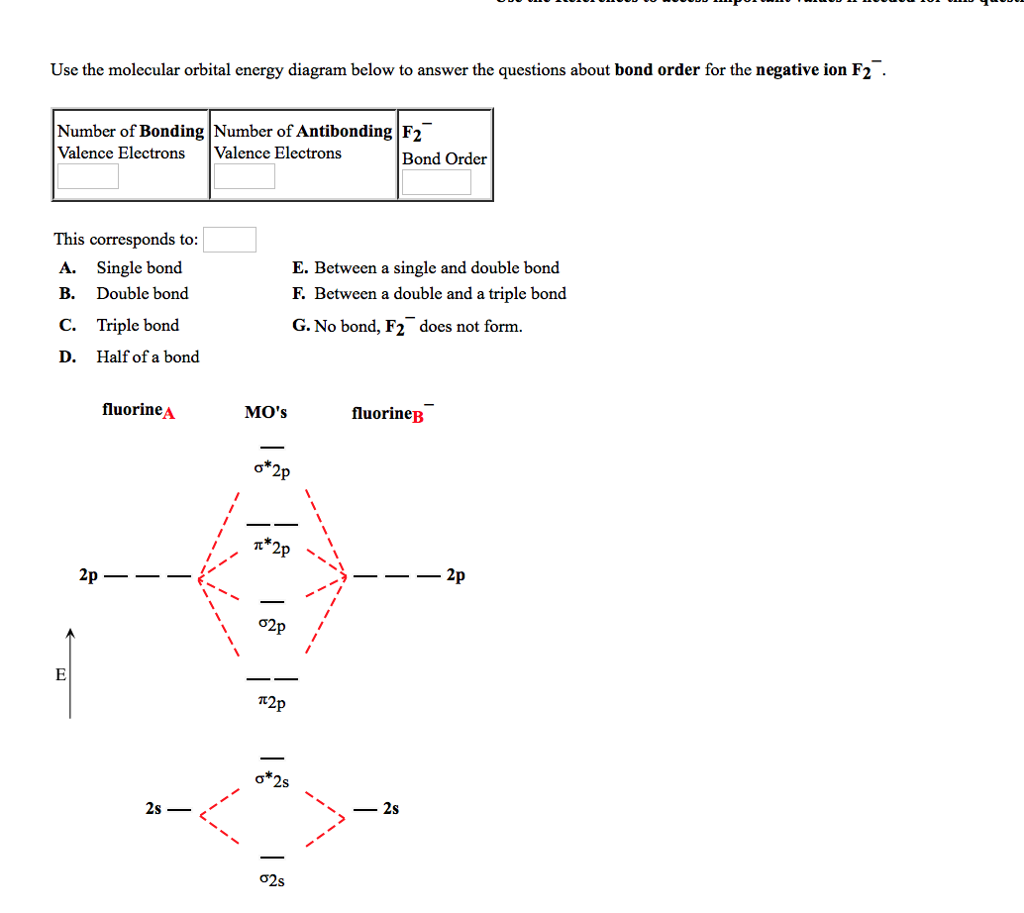 Solved Use The Molecular Orbital Energy Diagram Below To
Solved Use The Molecular Orbital Energy Diagram Below To
What Is The Molecular Orbital Diagram For O2 And O2 Ions Quora
Draw The Molecular Orbital Diagram Of N2 Also Find Its Bond Order
 Theories Of Chemical Bonding Ppt Video Online Download
Theories Of Chemical Bonding Ppt Video Online Download
Molecular Orbital Diagram Of Ion Free Wiring Diagram For You
 Mo Bonding In F2 And O2 Chemistry Libretexts
Mo Bonding In F2 And O2 Chemistry Libretexts
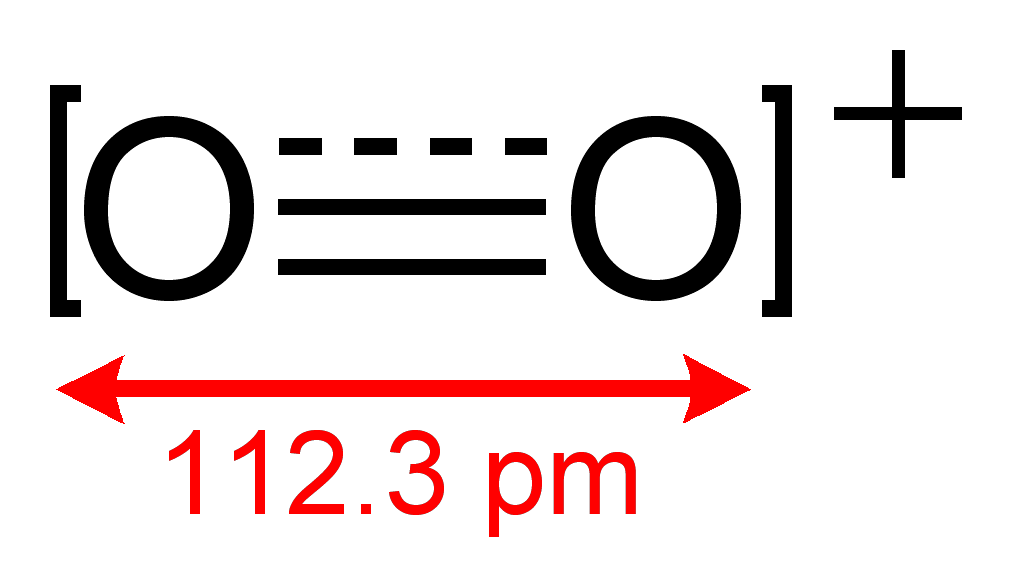
0 Response to "O2 Molecular Orbital Diagram"
Post a Comment