The Following Is A Diagram Of Energy States And Transitions In The Hydrogen Atom
The following is a diagram of energy states and transitions in the hydrogen atom. The formula defining the energy levels of a hydrogen atom are given by the equation.
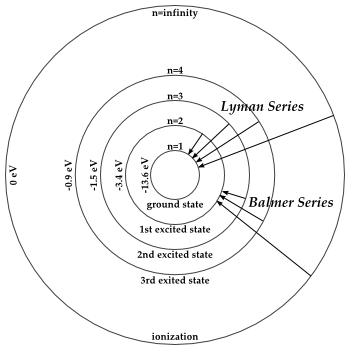 Transitions Hydrogen Energy Levels Naap
Transitions Hydrogen Energy Levels Naap
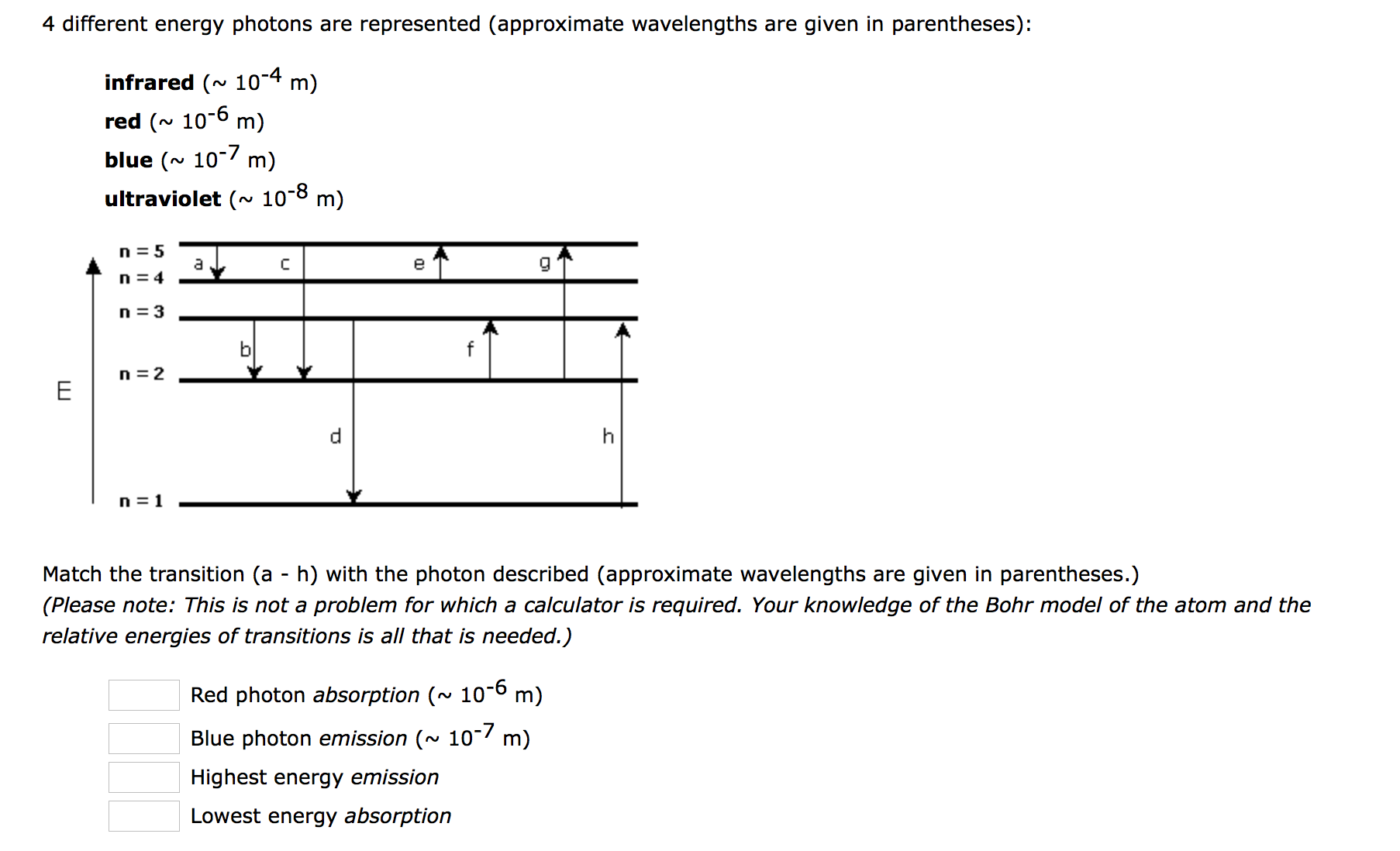
The labeled transitions a through e represent an electron moving between energy levels.
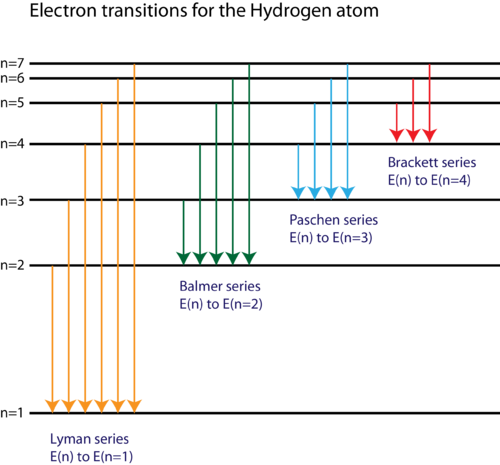
The following is a diagram of energy states and transitions in the hydrogen atom. The following is a diagram of energy states and transitions in the hydrogen atom. The labeled transitions a through e represent an electron moving between energy levels. The diagram for hydrogen is shown above.
The energy is expressed as a negative number because it takes that much energy to unbind ionize the electron from the nucleus. Suppose that an electron in a hydrogen atom absorbs 102 ev of energy so that it moves from level 1 to level 2. Match each of the responses below with the correct arrow from the figure.
The emission line with the highest energy. 4 a the emission line with the shortest wavelength. Its often helpful to draw a diagram showing the energy levels for the particular element youre interested in.
The following is a diagram of energy states and transitions in the hydrogen atom. The 2s and 2p states are found to differ a small amount in what is called the lamb shift. And even the 1s ground state is split by the interaction of electron spin and nuclear spin in what is called hyperfine structure.
For a single electron instead of per mole the formula in ev electron volts is also widely used. The absorption line with the shortest wavelength. The 2p level is split into a pair of lines by the spin orbit effect.
E e0n2 where e0 136 ev 1 ev 160210 19 joules and n 123 and so on. Figure 1 if an electron at level 1 in a hydrogen atom absorbs 102 ev of energy it moves to level 2. The emission line with the shortest wavelength.
B the electron returns to level 1 by emitting an ultraviolet photon with 102 ev of energy. 1 b the absorption line with the longest wavelength. The diagram represents energy levels in a hydrogen atom.
Electron shells and energy levels. The energy level of the electron of a hydrogen atom is given by the following formula where n denotes the principal quantum number. The following diagram represents energy levels in a hydrogen atom.
Match each of the responses below with the correct arrow from the figure. For hydrogen the ionization energy 136ev. The n 1 state is known as the ground state while higher n states are known as excited states.
Energy level diagrams and the hydrogen atom. The ionization energy of an atom is the energy required to remove the electron completely from the atomtransition from ground state n 0 to infinity n. 3 d the absorption line with the highest energy.
If none are correct enter none. When an excited electron returns to a lower level it loses an exact amount of energy by emitting a photon. 4 c the emission line with the highest energy.
Hydrogen energy level plot. Match each arrow with the correct response below.
Hydrogen Atom For Each Of The Following Electronic Transitions In
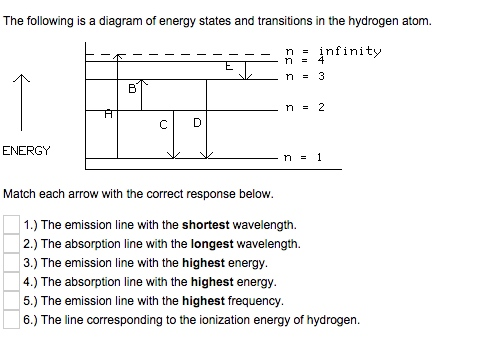 The Following Is A Diagram Of Energy States And Tr Chegg Com
The Following Is A Diagram Of Energy States And Tr Chegg Com
 Transition Element Chemical Element Britannica Com
Transition Element Chemical Element Britannica Com
Hydrogen Atom Hydrogen Atom Transitions Ionization
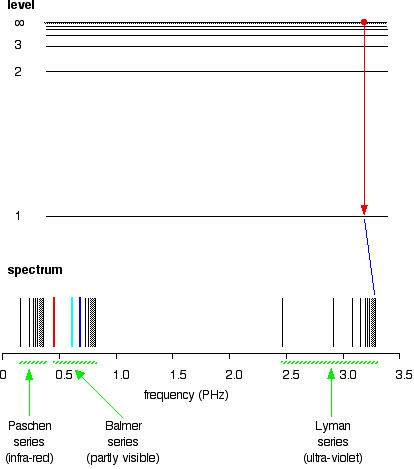 Hydrogen S Atomic Emission Spectrum Chemistry Libretexts
Hydrogen S Atomic Emission Spectrum Chemistry Libretexts
Types Of Stars Absorption Lines
 Bohr Model Of The Hydrogen Atom Electron Transitions Atomic Energy
Bohr Model Of The Hydrogen Atom Electron Transitions Atomic Energy
 Emission Spectrum Of The Hydrogen Atom Introduction To Chemistry
Emission Spectrum Of The Hydrogen Atom Introduction To Chemistry
 Transitions Between Two Levels Of The Small Hydrogen Atom As A
Transitions Between Two Levels Of The Small Hydrogen Atom As A
Hydrogen Atom Energy Levels And Transitions

 Solved Below Is The Energy Level Diagram Representing The
Solved Below Is The Energy Level Diagram Representing The
 Spectral Lines Of Hydrogen Chemistry For Non Majors
Spectral Lines Of Hydrogen Chemistry For Non Majors
 Hydrogen Atom Energy Levels N 3 And N 2 With Linear Dashed Lines
Hydrogen Atom Energy Levels N 3 And N 2 With Linear Dashed Lines
The Hydrogen Balmer Series And Rydberg Constant
Relativistic Energy Levels For Hydrogen Atom Wolfram

 Energy Level Diagram For Hydrogen Mini Physics Learn Physics Online
Energy Level Diagram For Hydrogen Mini Physics Learn Physics Online



0 Response to "The Following Is A Diagram Of Energy States And Transitions In The Hydrogen Atom"
Post a Comment