Create The Atomic Orbital Diagram For Chlorine
Create the atomic orbital diagram for chlorine. Ch3oh ch3li ch3f ch3nh2 ch 4.
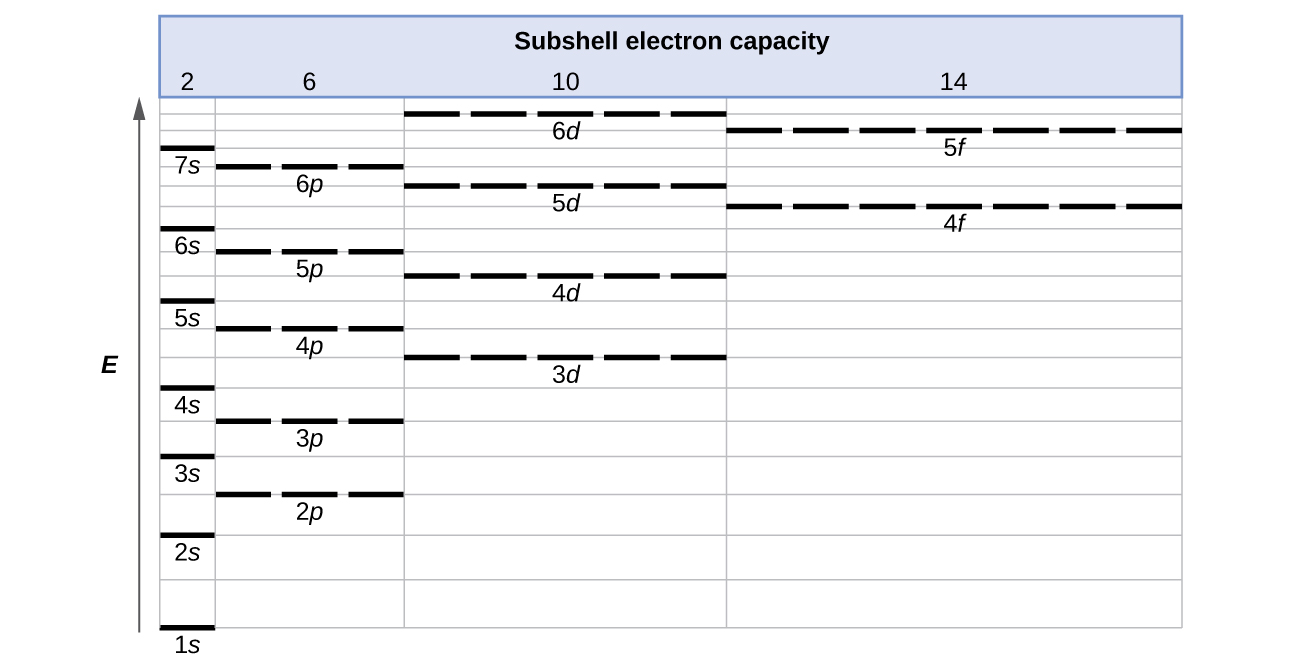 6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
Draw orbital diagrams for the following elements.
Create the atomic orbital diagram for chlorine. In a chlorine atom which subshells contain valence electrons. Create the atomic orbital diagram for nitrogen. 3p 3s 2p 2s or 1s.
In a chlorine atom which subshells contain vale. The p orbital can hold up to six electrons. Draw the atomic orbital diagram for chlorine.
Create the atomic orbital diagram for chlorine. In a chlorine atom which subshells contain vale. Create the atomic orbital diagram for chlorine.
Write the electron configuration full and in core notation for the following ions. How to create a beautiful bedroom download pdf so2 atomic orbital diagram for free at this site. Since 1s can only hold two electrons the next 2 electrons for chlorine go in the 2s orbital.
Show transcribed image text create the atomic orbital diagram for chlorine. Draw the atomic orbital diagram for chlorine. The next six electrons will go in the 2p orbital.
Write the electron configuration full and in core notation. In a chlorine atom which subshells contain valance electrons. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 kr ar 3d 10 4s 2 4p 6 2.
Construct the orbital diagram of each atom or ion. Rank the following compounds according to increasing positive character of the carbon atom. Commercial quantities are produced by electrolysis of aqueous sodium chloride seawater or brine from salt mines.
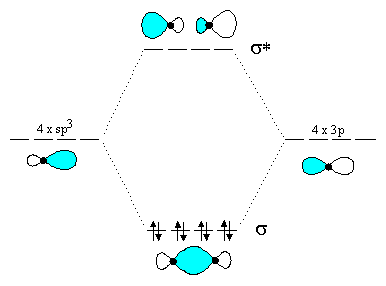
In writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. An orbital diagram is a sketch which shows electron population in atomic orbitals with the electrons spin indicated by up and down arrows. The superposition of the two 1s atomic orbitals leads to the formation of the σ and σ molecular orbitals.
Predict the approximate bond angle in the following molecule. How many electrons does a fe atom have in its 3. If the two 1s orbitals are not in phase a node between them causes a jump in energy the σ orbital.
Salt sodium chloride nacl is its most common compound. Two atomic orbitals in phase create a larger electron density which leads to the σ orbital. Draw orbital diagrams for the following elements.
Ionization Energy And Electron Affinity
 Chlorine A Multi Tasking Molecule
Chlorine A Multi Tasking Molecule
Molecular Orbital Diagram Wikipedia
 Electronic Configuration The Atom Siyavula
Electronic Configuration The Atom Siyavula
An Integrative Synthetic Spectroscopic And Computational Study Of
What Is The Ground State Configuration Of Chlorine Socratic
:max_bytes(150000):strip_icc()/Technetium-58b601d33df78cdcd83d1289.jpg) Atom Diagrams Electron Configurations Of The Elements
Atom Diagrams Electron Configurations Of The Elements

 Dublin Schools Lesson Orbital Diagrams And Electron Configurations
Dublin Schools Lesson Orbital Diagrams And Electron Configurations
Why Is Bcl3 An Electron Deficient Compound And Why Does It Have A
 Answers To Problem Set 2 Chem2o6
Answers To Problem Set 2 Chem2o6
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 Section 11 4 Electron Configurations And Atomic Properties 1 To
Section 11 4 Electron Configurations And Atomic Properties 1 To
How Many Valence Electrons Are In An Atom Of Chlorine Socratic
Answer Key Problem Set 11 Full
 Chlorine Electron Configuration Youtube
Chlorine Electron Configuration Youtube



0 Response to "Create The Atomic Orbital Diagram For Chlorine"
Post a Comment