Draw The Octahedral Crystal Field Splitting Diagram For Each Metal Ion
Construct the octahedral crystal field splitting diagram for the metal in each species. Lecture 9 crystal field theory for octahedral.
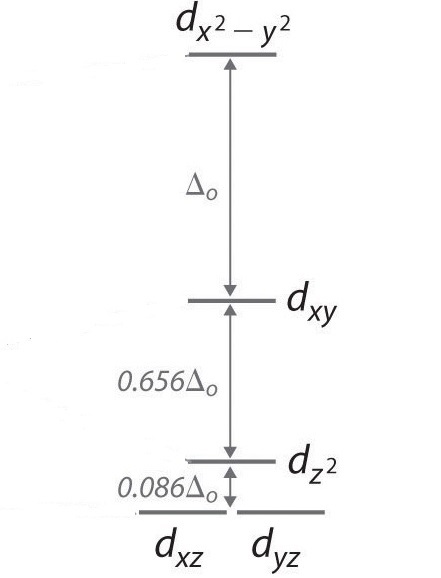 Crystal Field Theory Chemistry Libretexts
Crystal Field Theory Chemistry Libretexts
Cfse the stability that results from placing a transition metal ion in the crystal field generated by a set of ligands.
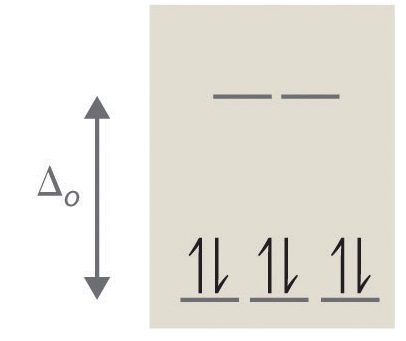
Draw the octahedral crystal field splitting diagram for each metal ion. Crystal field splitting energy 4. Blogadmin 2 hours ago question leave a comment 1 views. How to draw the crystal field splitting diagram 2.
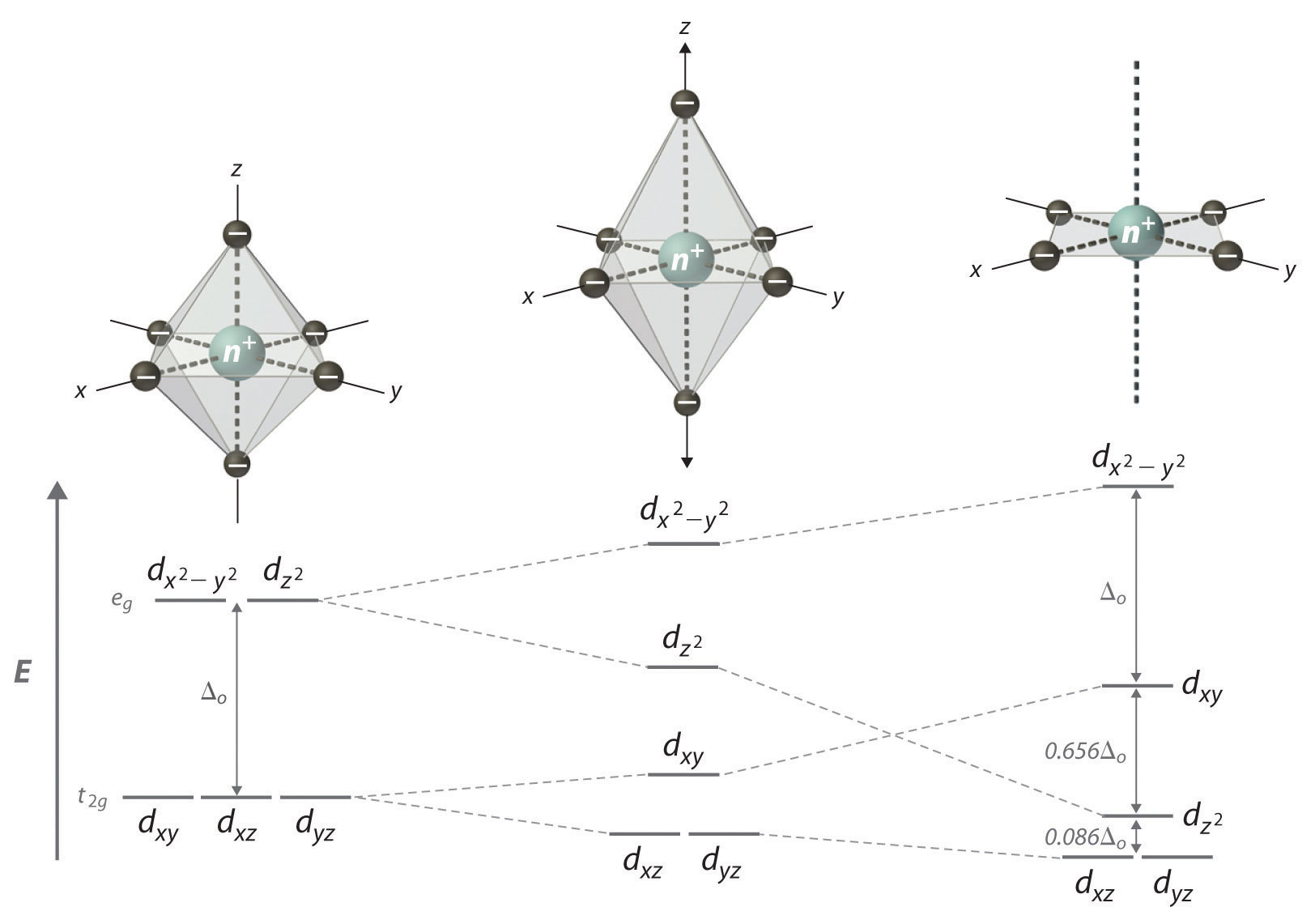
The d z. The dx2 y2 and dz2 orbitals on the metal ion at the center of the cube lie between the ligands and the dxy dxz and dyz orbitals point toward the ligands. In order for low spin splitting to occur the energy cost of placing an electron into an already singly occupied orbital must be less than the cost of placing the additional electron into an e g orbital at an energy cost of δ.
Draw the octahedral crystal field splitting diagram for each metal ion. Draw the octahedral crystal field splitting diagram for each metal ion. Home question construct the octahedral crystal field splitting diagram for the metal in each species.
The octahedral splitting energy is the energy difference between the t 2g and e g orbitals. For transition metal complexes called crystal field theory. This diagram shows the field splitting of a metal with ligands in an octahedral configuration.
The thick horizontal lines represent atomic orbitals of the metal left and ligands right. How to determine the oxidation state of the transition metal in a. So the ion febr 6 3 again with five d electrons would have an octahedral splitting diagram where all five orbitals are singly occupied.
Cr3 cu2 mn3 high spin. As a result the splitting observed in a tetrahedral crystal field is the opposite of the splitting in an octahedral complex. In an octahedral field the t.
Electrons in d orbitals. Lecture 7 crystal field theory for octahedral complexes boats and propellers. The central transition metal atom or ion is grey the six ligands are red and the orbitals are yellow.
Depending on the energy gap between the d orbitals in an octahedral geometry the complex may be high spin weak field ligands or low spin strong field ligands. Crystal field theory. Weak field vs strong field diagrams 3.
The resulting d orbital splitting diagram for. Cr3 cu2 mn3 high spin.
 Crystal Field Theory Chemistry Libretexts
Crystal Field Theory Chemistry Libretexts
 Octahedral Crystal Field Splitting Diagram
Octahedral Crystal Field Splitting Diagram
Chemistry The Central Science Chapter 24 Section 5
Chemistry The Central Science Chapter 24 Section 5
 Draw Figure To Show Splitting Of D Orbitals In An Octahedral Crystal
Draw Figure To Show Splitting Of D Orbitals In An Octahedral Crystal
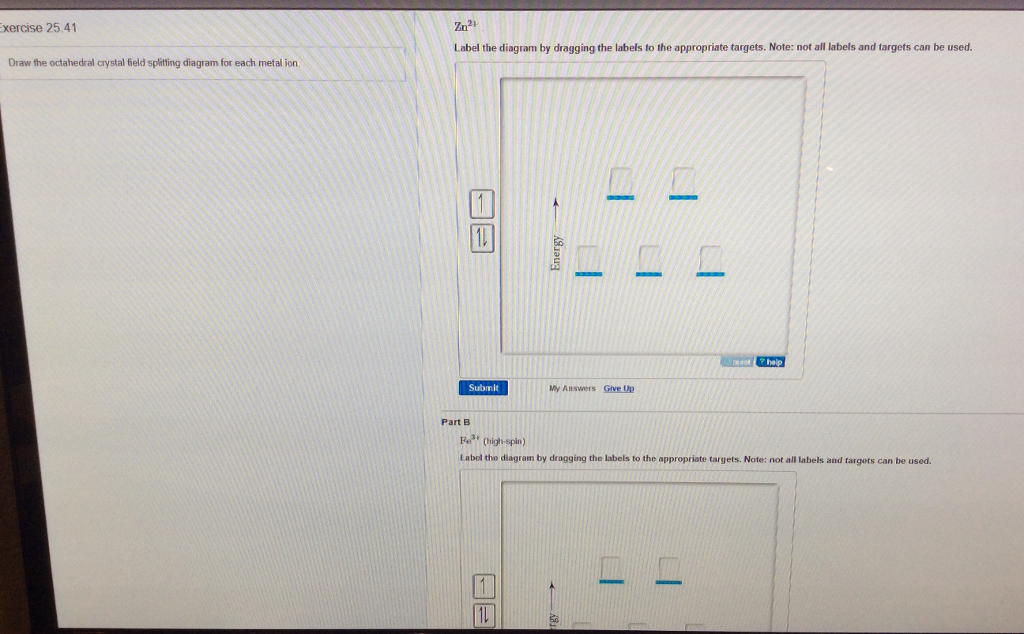 Solved Please Answer Each Of The Following As Stated In T
Solved Please Answer Each Of The Following As Stated In T
 Solution For Draw Figure To Show The Splitting Of D Orbitals In An
Solution For Draw Figure To Show The Splitting Of D Orbitals In An
 Crystal Field Theory Wikipedia
Crystal Field Theory Wikipedia
 Introduction To Inorganic Chemistry Coordination Chemistry And
Introduction To Inorganic Chemistry Coordination Chemistry And
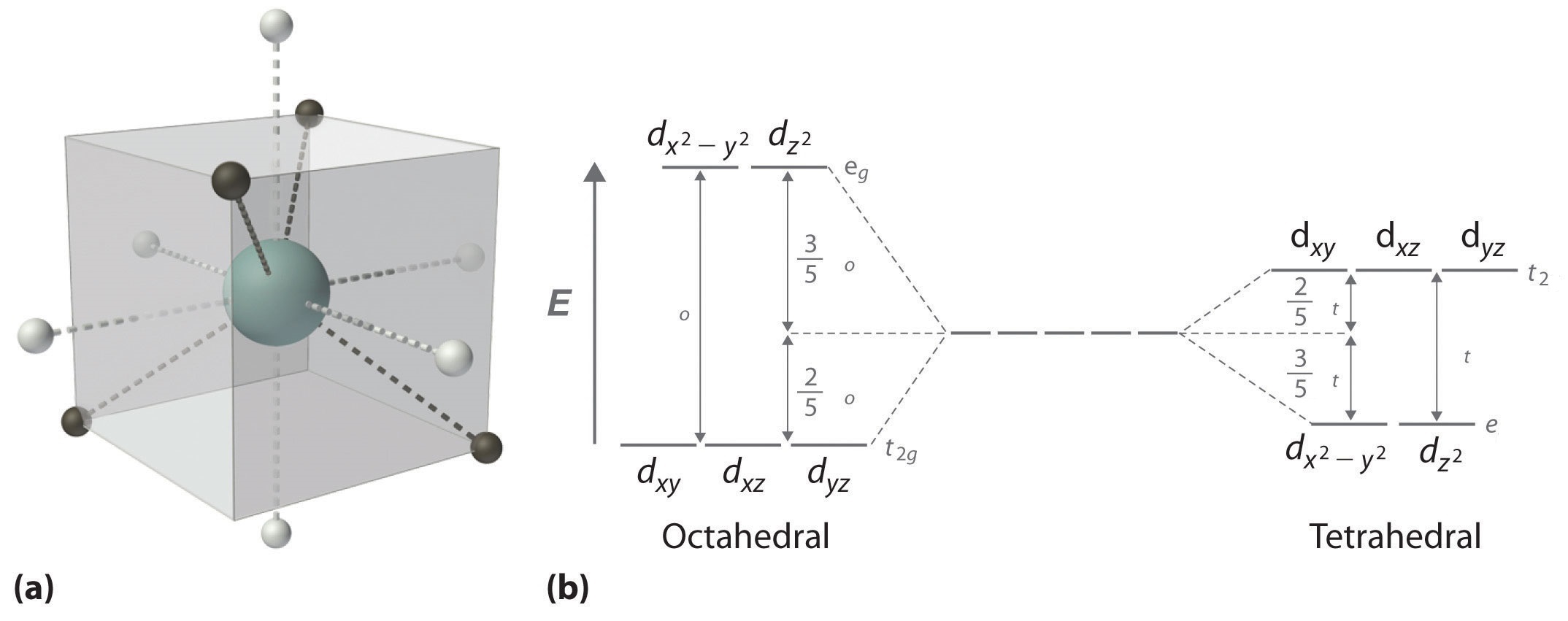 Crystal Field Theory Chemistry Libretexts
Crystal Field Theory Chemistry Libretexts
 Draw Figure To Show The Splitting Of D Orbitals In An Octahedral
Draw Figure To Show The Splitting Of D Orbitals In An Octahedral
 Introduction To Inorganic Chemistry Coordination Chemistry And
Introduction To Inorganic Chemistry Coordination Chemistry And
Questions Answers On Coordination Chemistry D Ray
 Why Does The Splitting Of D Orbital Occur In Octahedral And
Why Does The Splitting Of D Orbital Occur In Octahedral And

0 Response to "Draw The Octahedral Crystal Field Splitting Diagram For Each Metal Ion"
Post a Comment