Use The Orbital Filling Diagram To Show The Electron Configuration Of Phosphorus P
In this video well use the electron configuration chart to help us write the notation for phosphorus. Be sure to label the subshells in order of energy with the lowest energy subshell at the bottom and the highest energy drag the appropriate labels to their respective targets.
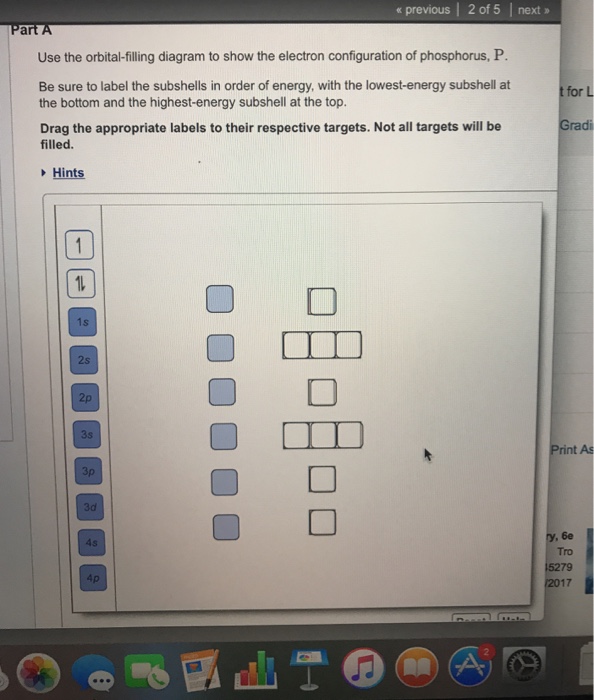
Be sure to arrange the subshells in order of energy with the lowest energy subshell at the bottom and the highest energy subshell at the top.

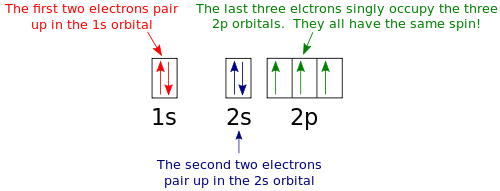
Use the orbital filling diagram to show the electron configuration of phosphorus p. Show the orbital filling diagram for n nitrogen. The 3p electrons are in three different orbitals and have the same spin. The p orbital can hold up to six electrons.
Be sure to arrange the subshells in order of e. The orbital diagram for phosphorus consists of five electrons in the third shell eight in the second and two in the first shell closest to the nucleus. Not all targets will be filled.
Show the orbital filling diagram for br bromine. Stack the subshells in order of energy with the lowest energy subshell at the bottom and the highest energy subshell at the top. Because the atomic number is equivalent to the number of electrons the standard electron configuration can be used to draw the orbital diagram.
Show the electron configuration of phosphorusp. Since 1s can only hold two electrons the next 2 electrons for phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital.
Stack the subshells in order of energy with the lowest energy subshell at the bottom and the highest energy subshell at the top. Electron configuration element ne 3s 2 magnesium ar 4s 2 3d 5 manganese kr 5s 2 4d 10 5p 3 antimony 5. Use the periodic table to identify the neutral atoms having the following electron configurations.
Note that the last term in the phosphorus electron configuration will be 1s2 2s2 2p6 3s2 3p3. Show transcribed image text use the orbital filling diagram to show the electron configuration of phosphorus p. In writing the electron configuration for phosphorus the first two electrons will go in the 1s orbital.
With an atomic number of 15 two electrons. Show the electron configuration of phosphorusp. Stack the subshells in order of energy with the lowest energy subshell at the bottom and the highest energy subshell at the top.
Give the complete ground state electron configuration for silicon si.
 How To Write The Shorthand Electron Configuration For Lead Sciencing
How To Write The Shorthand Electron Configuration For Lead Sciencing
Chapter 7 Electron Configurations And The Properties Of Atoms 7 1
 High School Chemistry Orbital Configurations Wikibooks Open Books
High School Chemistry Orbital Configurations Wikibooks Open Books
 Electron Configuration Wikipedia
Electron Configuration Wikipedia
 Electron Configuration Chart Of Elements Science Trends
Electron Configuration Chart Of Elements Science Trends
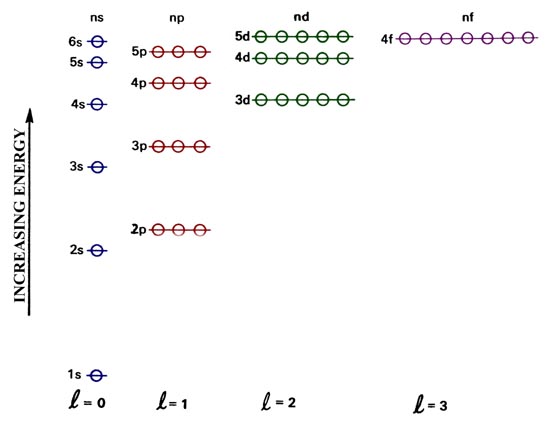 Many Electron Atoms The Electronic Basis Of The Periodic Table
Many Electron Atoms The Electronic Basis Of The Periodic Table
Chem4kids Com Phosphorus Orbital And Bonding Info
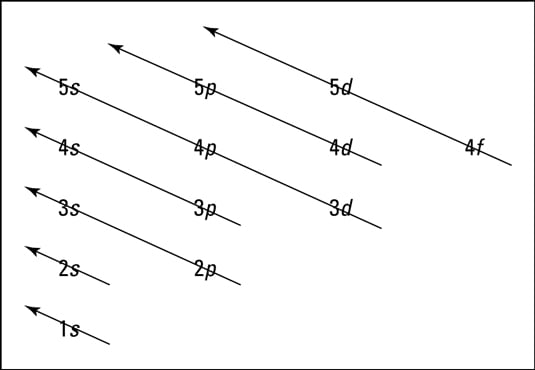 How To Represent Electrons In An Energy Level Diagram Dummies
How To Represent Electrons In An Energy Level Diagram Dummies
Quantum Numbers And Electron Configurations
Electron Configurations How To Write Out The S P D F Electronic
 Electron Configuration Wikipedia
Electron Configuration Wikipedia
Arrangements Of Electrons In The Orbitals Of An Atom Is Called Its
 High School Chemistry Orbital Configurations Wikibooks Open Books
High School Chemistry Orbital Configurations Wikibooks Open Books
 Electron Configuration Revolvy
Electron Configuration Revolvy
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry

0 Response to "Use The Orbital Filling Diagram To Show The Electron Configuration Of Phosphorus P"
Post a Comment