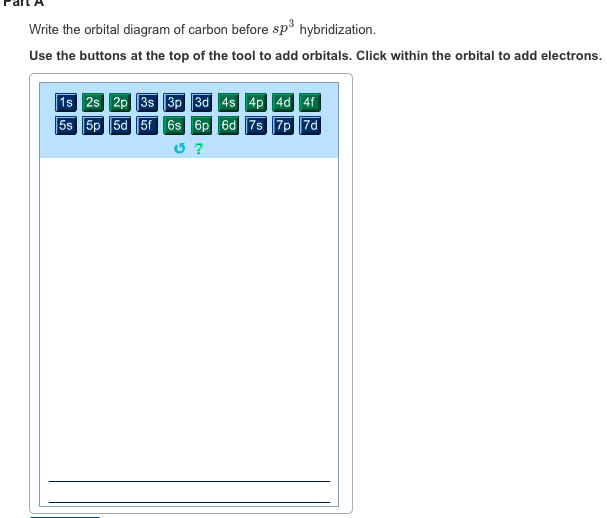
Write The Orbital Diagram Of Carbon Before Sp3 Hybridization
Write the orbital diagram of carbon before sp hybridization. There is also one half filled unhybridized 2p z orbital on each carbon perpedicular to the plane of sp 2 hybrid orbitals.
 What Is The Hybridization For C2h6 My Teacher Wrote Sp S Sigma Bond
What Is The Hybridization For C2h6 My Teacher Wrote Sp S Sigma Bond
Chemistry quantum mechanical model of the atom orbitals and probability patterns.

Write the orbital diagram of carbon before sp3 hybridization. According to hunds rule each orbital must contain one electron each with the same spin before adding a second electron. So this is how the hydrogen orbital and the carbon orbitals get mixed. So lets get some more room.
The angle between them is 180o making co 2 a linear molecule as predicted by vespr. Write the orbital diagram of carbon before sp 3 hybridization. If each carbon is sp three hybridized that means each carbon is gonna have four sp three hybrid orbitals.
Click within the orbital to add electrons. The hydrogens 1s orbital bonds with well each of the hydrogens 1s orbital bonds with each of the carbons sp3 orbitals. Each of the carbons in ethane has four single bonds so each carbon in ethane is sp three hybridized so let me go ahead and put sp three hybridized here so lets go ahead and draw the picture with the orbitals.
You can read more about sp3 hybridization here. The p orbitals are equal in energy and said to be degenerate. Because the 2p orbital constitutes most of the sp3 orbitals 75 and because the 2p orbitals were higher in energy than the 2s orbitals the sp3 orbitals are closer in energy to the original 2p orbitals than to the original 2s orbital.
The two singly occupied p orbitals can be utilized for bonding to give methylene ch2 an unstable free radical figure 3. The ground state configuration of carbon is 1s2 2s2 2px1 2py1. How do you write the orbital diagram for carbon.
The resulting hybrid orbitals are called sp hybrids. It has a hydrogen here 1s orbital hydrogen here 1s orbital. Just so you get a little bit more notation.
Consider the electron configuration of a carbon atom. To form 2 hybrid molecular orbitals we need to mix 2 atomic orbitals an s orbital and a p orbital. So in the 2p orbitals there are two electrons each with the same spin.
The carbon atoms form a σ sp 2 sp 2 bond with each other by using sp 2 hybrid orbitals. The two unhybridized p orbitals on carbon form p bonds to the oxygen atoms. Use the buttons at the top of the tool to add orbitals.
Bonding In Methane Sp3 Hybridisation
Organic Chemistry Applied Physics Learning Centre
 Solved Write The Orbital Diagram Of Carbon Before Sp Hybr
Solved Write The Orbital Diagram Of Carbon Before Sp Hybr
 Hybridisation Definition Types Rules Examples Videos Solved
Hybridisation Definition Types Rules Examples Videos Solved
 Chemical Bonding Of H2o Wikipedia
Chemical Bonding Of H2o Wikipedia
 The Trouble With The Aufbau Principle Feature Education In Chemistry
The Trouble With The Aufbau Principle Feature Education In Chemistry
Orbital Diagram Of Sp3 Wiring Diagram Schematics
 Using Orbital Hybridization And Valence Bond Theory To Predict
Using Orbital Hybridization And Valence Bond Theory To Predict
Vsepr Theory A Closer Look At Chlorine Trifluoride Clf3 Henry Rzepa
Ch310m Ch318m Iverson Mo And Vb Theory

 Hybridization Hybrid Orbitals Forming Molecular Orbitals
Hybridization Hybrid Orbitals Forming Molecular Orbitals
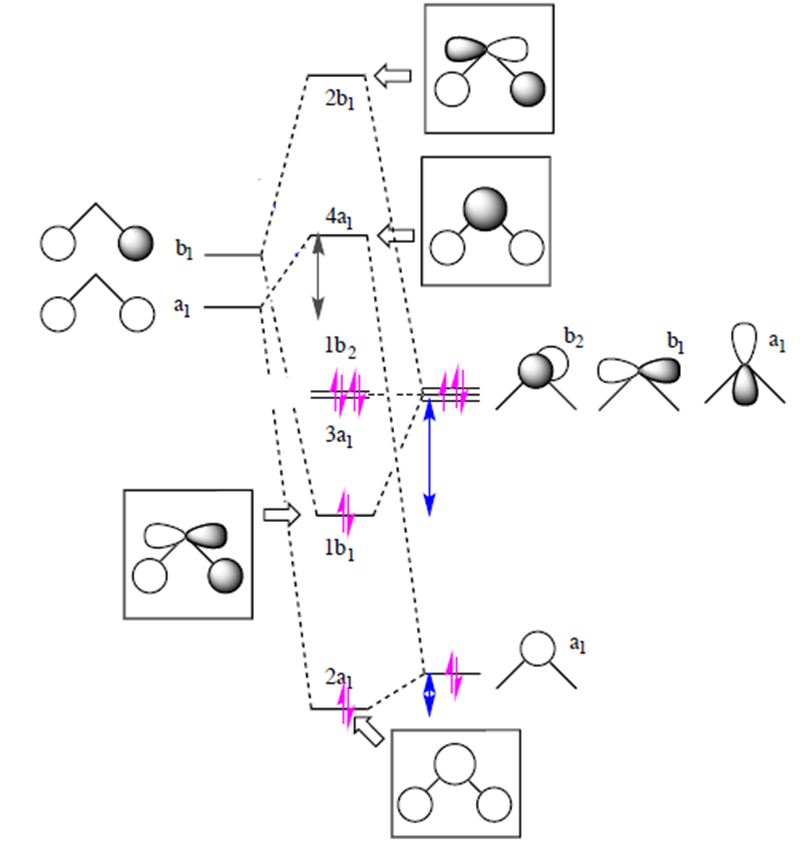 Chemical Bonding Of H2o Wikipedia
Chemical Bonding Of H2o Wikipedia
Bonding Orbitals In Formaldehyde
Valence Bond Theory And Hybrid Orbitals Introductory Chemistry
 Solved Write Orbital Diagrams Boxes With Arrows In Them
Solved Write Orbital Diagrams Boxes With Arrows In Them
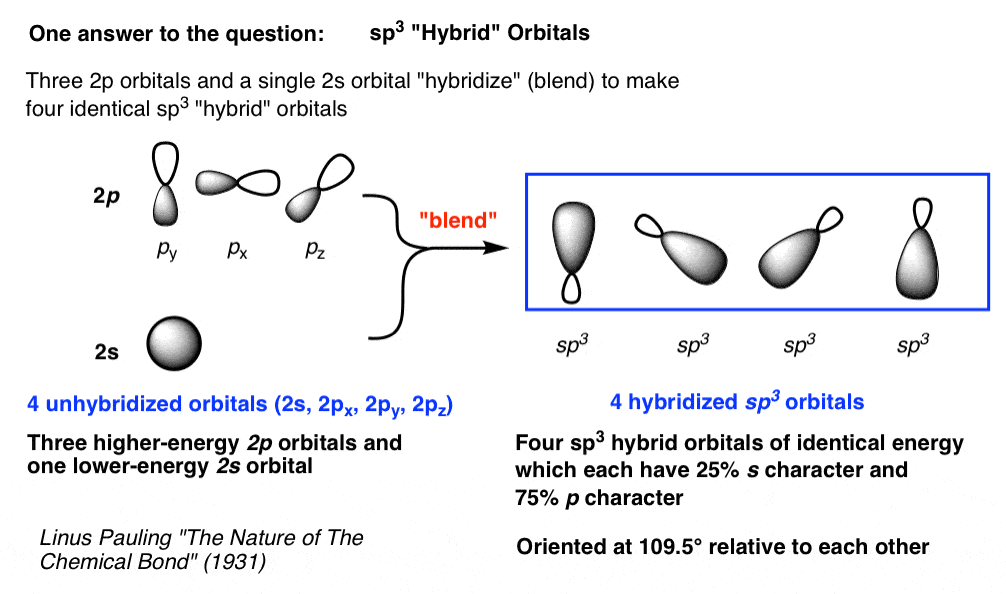 Hybrid Orbitals Master Organic Chemistry
Hybrid Orbitals Master Organic Chemistry
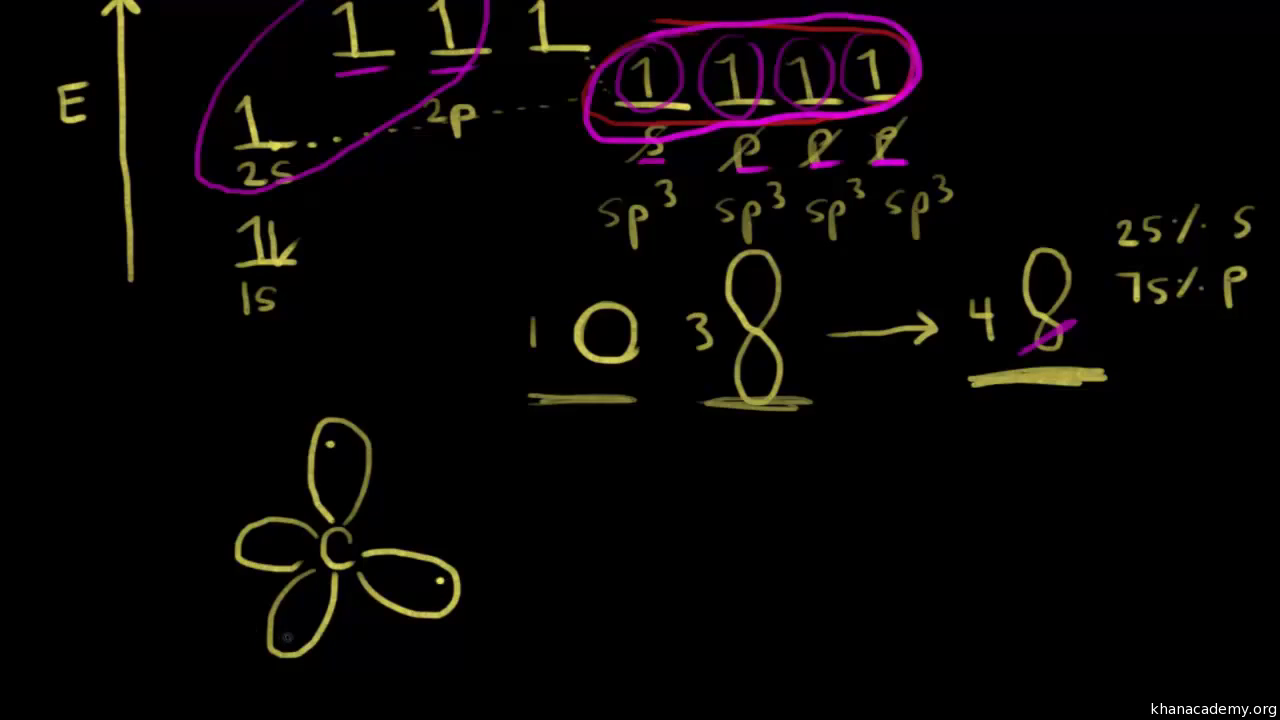 Sp Hybridization Hybrid Orbitals Chemical Bonds Video Khan
Sp Hybridization Hybrid Orbitals Chemical Bonds Video Khan
0 Response to "Write The Orbital Diagram Of Carbon Before Sp3 Hybridization"
Post a Comment