Electron Dot Diagram For Co2
I also go over hybridization shape and bond angles. But why does it stop there.
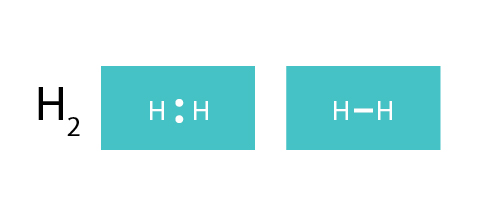 H2 Dot Diagram 3 2 Stromoeko De
H2 Dot Diagram 3 2 Stromoeko De
There is an easy way and a formal way to draw the lewis structure of co 2 carbon dioxide.
Electron dot diagram for co2. So lets multiply that together there. The structures do not look like the lewis dot structure for co2 where the carbon does not have any lone pair electrons on the carbon. So i know that the lewis dot structure for co2 is o c o the os have two pairs of 2 dots on each one.
The lewis structure for carbon dioxide. Which of these molecules is polar. In the formal way we find how many electrons we have step 1 how many each atom needs step 2 how many of those are bonding step 3 4 and how many are lone pairs step 5.
Carbon cis at the center. On the periodic table carbon is in group 4 or 14 sometimes. Carbon is in group 4 and oxygen is in group 6.
It decides molecular geometry and the electron group geometry. Co2 bond angles are 180 degrees. The carbon is sp hybridized and oxygens are.
Analyze bond angles and bonding pairs. So we have 12 plus 4 16 total valence electrons. The lewis structure of co2 looks something like that.
And then oxygen is in group 6 or 16. Lewis structures can also be drawn for ions. Why isnt there three lines and o only has one pair or 2 dots.
Co2 lewis dot structure. Why is there a difference in bonding angles between water and carbon dioxide. Vsepr theory means valence shell electron pair repulsion theory.
After that the application of vsepr theory takes place with the lewis structure. Were going to do the lewis structure for co2 carbon dioxide. In these cases the entire structure is placed in brackets and the charge is written as a superscript on the upper right outside of the bracket.
Lets draw the structure. This diagram shows the conceptual stages of drawing the lewis structure for a molecule of carbon dioxide co2. Draw the lewis dot structure of co2 and h2o.
The notable exceptions are the compounds cn cyanide and co carbon monoxide which the bond is polar due to the unequal sharing of electrons between the two atoms in the structure. But we have two of them. In the formation of co 2 there are two particles.
There is a double bond between each oxygen and carbon. I quickly take you through how to draw the lewis structure of co2 carbon dioxide.
 What Is The Lewis Structure Of Co3 2 Quora
What Is The Lewis Structure Of Co3 2 Quora
E Dot Diagram Co Wiring Diagrams
Electron Dot Diagram Lewis Diagram For Co2 Daytonva150 Lewis
 Lewis Diagram Hobr Wiring Schematic Diagram
Lewis Diagram Hobr Wiring Schematic Diagram
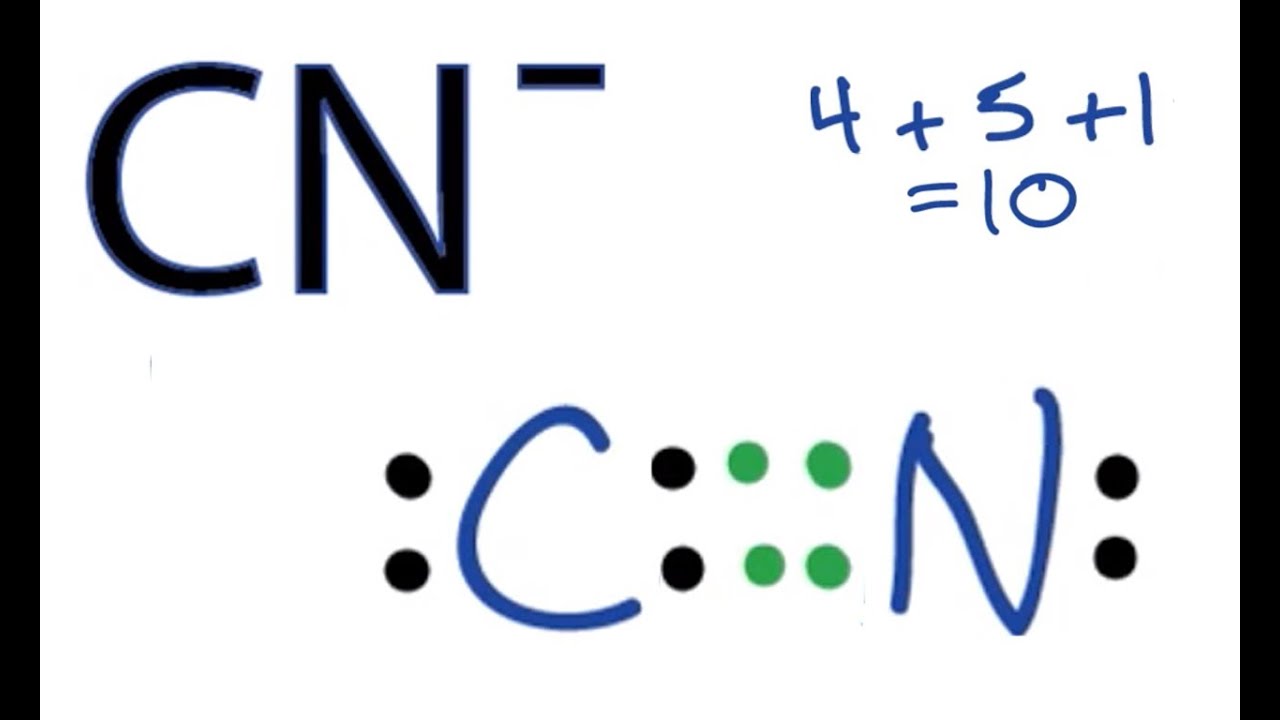 The Lewis Dot Structure For Co2 Makethebrainhappy
The Lewis Dot Structure For Co2 Makethebrainhappy
Lewis Dot Diagram For Co2 Awesome Ch3 Structure How To Draw The Of 8
Carbon Dioxide Electron Dot Diagram Fresh The Lewis Dot Structure
How Can I Draw A Lewis Dot Diagram For Carbon Dioxide Socratic
 How Many Pi Bonds Are There In Co 2 Socratic
How Many Pi Bonds Are There In Co 2 Socratic
 Lewis Dot Structure Co2 Unique Which Lewis Electron Dot Diagram Is
Lewis Dot Structure Co2 Unique Which Lewis Electron Dot Diagram Is
 Lewis Dot Structure Co2 Inspirational 9 7 The Shapes Of Molecules
Lewis Dot Structure Co2 Inspirational 9 7 The Shapes Of Molecules
Lewis Diagram Co2 Free Wiring Diagram For You
 Lewis Dot Structure Co2 Lovely Electron Domain Definition And Vsepr
Lewis Dot Structure Co2 Lovely Electron Domain Definition And Vsepr
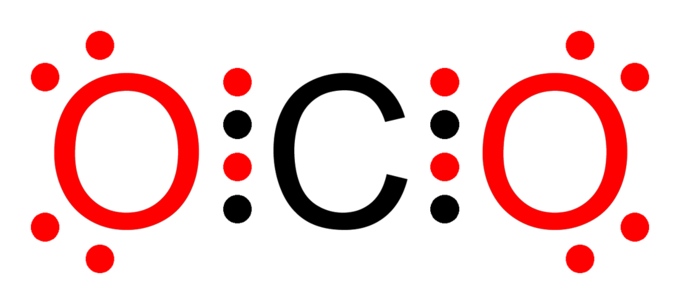 Exceptions To The Octet Rule Chemistry Master
Exceptions To The Octet Rule Chemistry Master
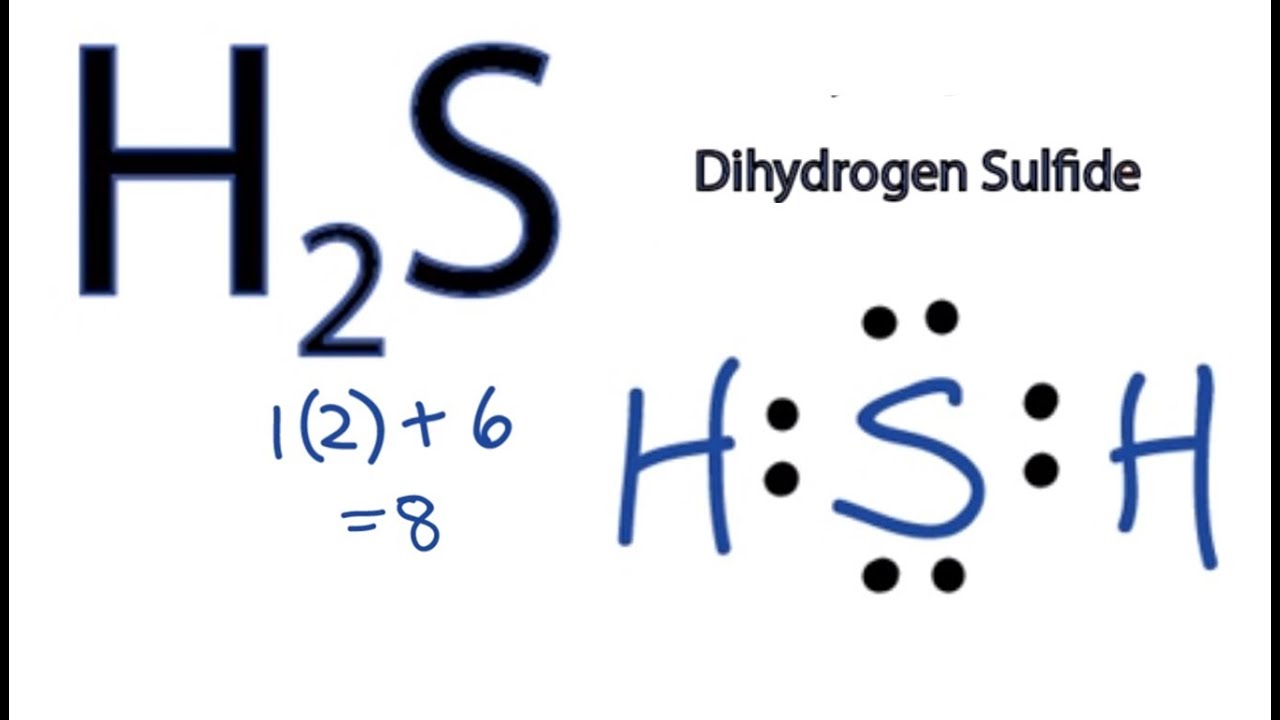 H2 Dot Diagram 3 2 Stromoeko De
H2 Dot Diagram 3 2 Stromoeko De
H H2o Dot Diagram H20 Lewis Oasissolutions Co
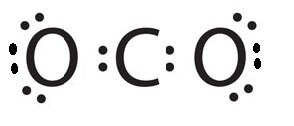 Co2 Molecular Geometry And Lewis Structure
Co2 Molecular Geometry And Lewis Structure
 Cl2 Lewis Diagram 18 11 Fearless Wonder De
Cl2 Lewis Diagram 18 11 Fearless Wonder De
 The Lewis Dot Structure For Co2 Makethebrainhappy
The Lewis Dot Structure For Co2 Makethebrainhappy
How Can I Draw A Lewis Dot Diagram For Carbon Dioxide Socratic
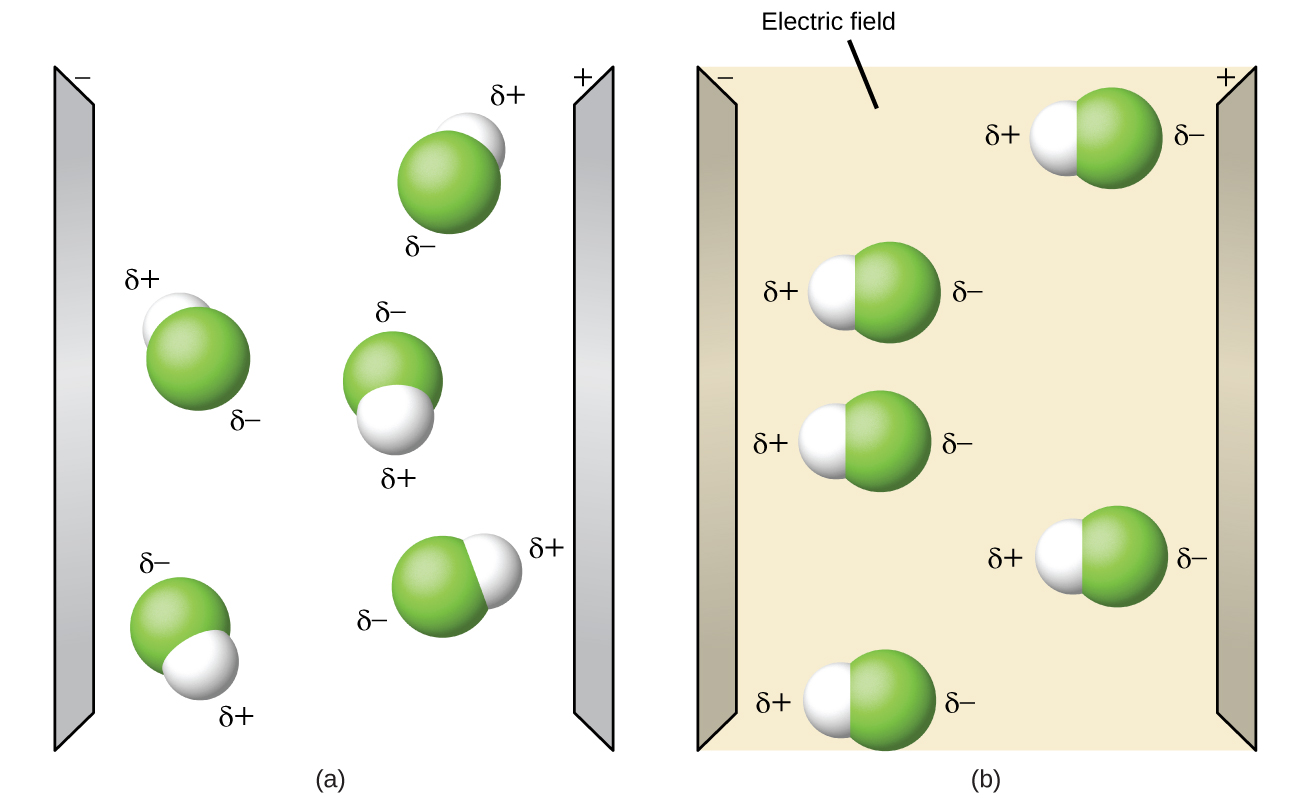 7 6 Molecular Structure And Polarity Chemistry
7 6 Molecular Structure And Polarity Chemistry
0 Response to "Electron Dot Diagram For Co2"
Post a Comment