Energy Diagram For Endothermic Reaction
The amount of energy put in to break these bonds is called. At the top of the curve the bonds in the reactants have been broken.
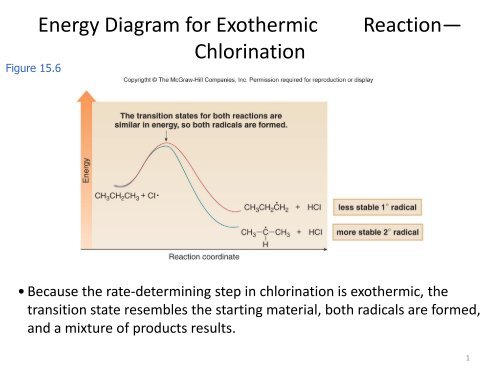 Energy Diagram For Exothermic Reaction Chlorination
Energy Diagram For Exothermic Reaction Chlorination
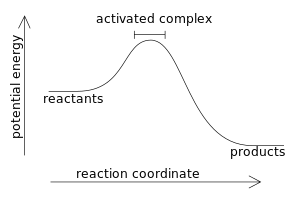
Energy diagrams for endothermic and exothermic reactions.

Energy diagram for endothermic reaction. This is because there will be an observable energy exchange between the chemicals and the surroundings. Energy profile diagrams for endothermic and exothermic reactions. An energy level diagram shows whether a reaction is exothermic or endothermic.
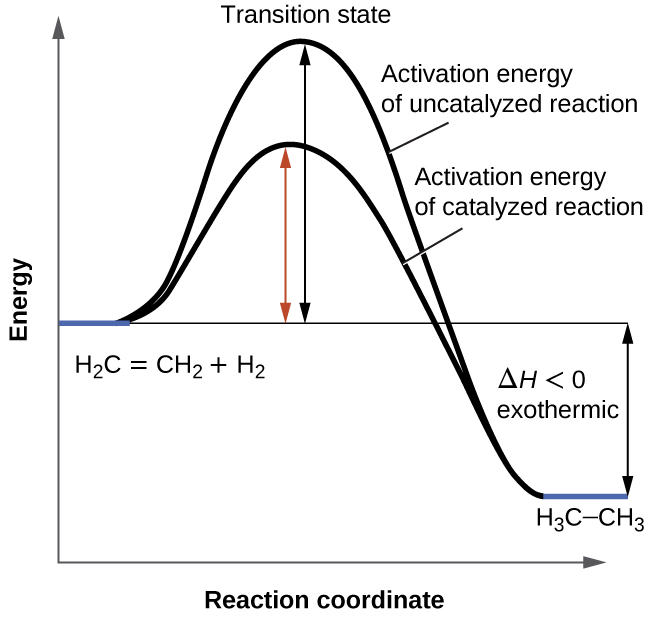
This energy diagram shows an exothermic reaction one in which energy is given off. This energy is given the symbol h and is different for different substances. It is difficult to measure the absolute energy of a substance but the change in energy during chemical reactions can be easily measured.
A reaction that takes in heat energy so the temperature goes down on a energy profile diagram is it a exothermic or endothermic reaction if the activation energy is small. In the case of an endothermic reaction the reactants are at a lower energy level compared to the productsas shown in the energy diagram below. More on pe diagrams.
Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Unfortunately for many reactions the real shapes of the energy profiles are slightly different from these and the rest of this page explores some simple differences. In the energy diagram for an endothermic reaction the energy of the products would be higher than that of the reactants.
Going from the reactants to the top of the curve we are going up the energy axis of the graph. If you had an endothermic reaction a simple energy profile for a non catalysed reaction would look like this. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions.
Energy flow endothermic reactions the reactants have less potential energy than do the products. Energy level diagram for an exothermic reaction is shown below. Endothermic reactions take in energy and the temperature of the surroundings decreases.
Energy is being put in to break bonds in the reactants. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. Energy reactants products exothermic reactions the reactants have more potential energy than the products have.
It also shows the effect of a catalyst on the forward and reverse activation energy. Energy must be input in order to raise the particles up to the higher energy level. In other words the products are less stable than the reactants.
Comparing Endothermic And Exothermic Potential Energy Diagrams
 Reaction Profile Energy Diagram Exothermic Moorpark College
Reaction Profile Energy Diagram Exothermic Moorpark College
Exothermic And Endothermic Changes
 Energy Level Diagram For An Endothermic Reaction Ap Chemistry
Energy Level Diagram For An Endothermic Reaction Ap Chemistry
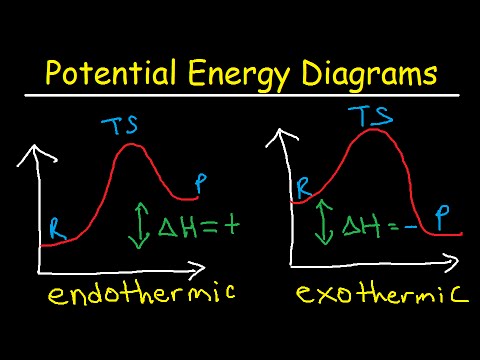 Potential Energy Diagrams Chemistry Catalyst Endothermic
Potential Energy Diagrams Chemistry Catalyst Endothermic

 Potential Energy Diagram Endothermic And Exothermic Ace Energy
Potential Energy Diagram Endothermic And Exothermic Ace Energy
 Activation Energy And The Activated Complex Energy And Chemical
Activation Energy And The Activated Complex Energy And Chemical
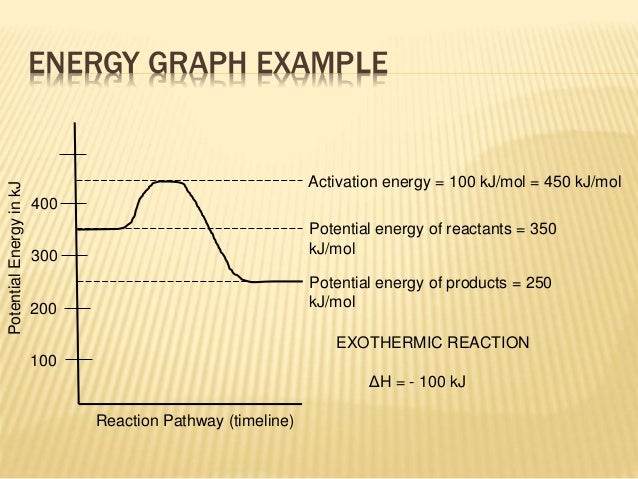 How To Read Potential Energy Diagrams
How To Read Potential Energy Diagrams
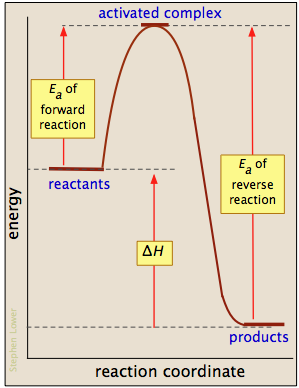 Basics Of Reaction Profiles Chemistry Libretexts
Basics Of Reaction Profiles Chemistry Libretexts
 Potential Energy Diagram Exothermic Ace Energy
Potential Energy Diagram Exothermic Ace Energy
Enthalpy Of Solution Chem 103 Lab Endothermic Reaction Energy
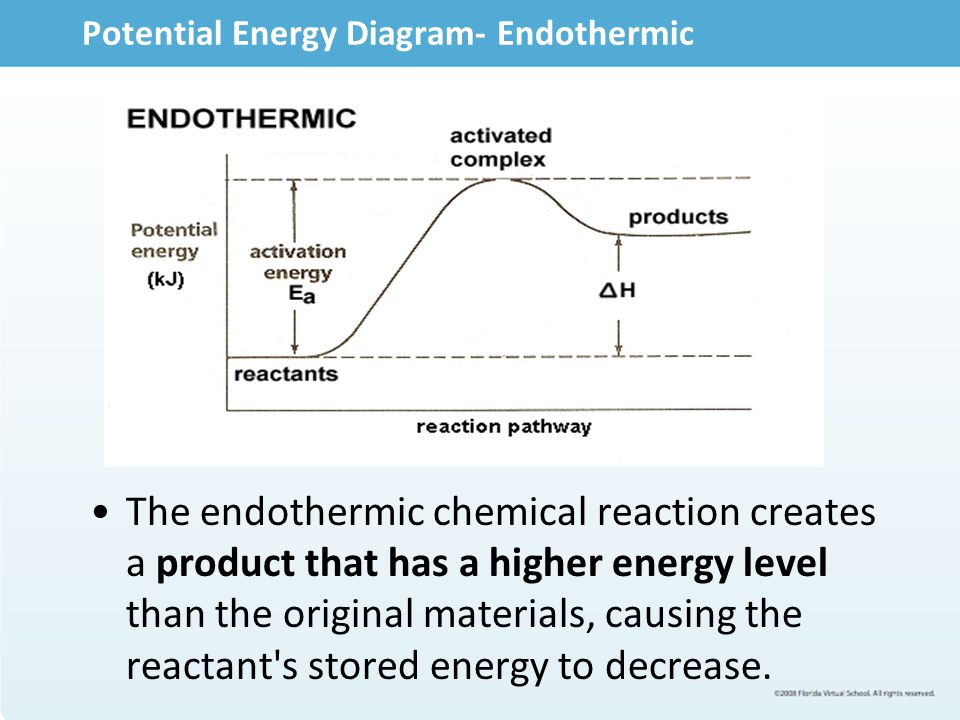 6 02 Chemlive Exothermic And Exothermic Reactions Ppt Video Online
6 02 Chemlive Exothermic And Exothermic Reactions Ppt Video Online
Organic Chemistry I New Reaction Energy Diagrams
0 Response to "Energy Diagram For Endothermic Reaction"
Post a Comment