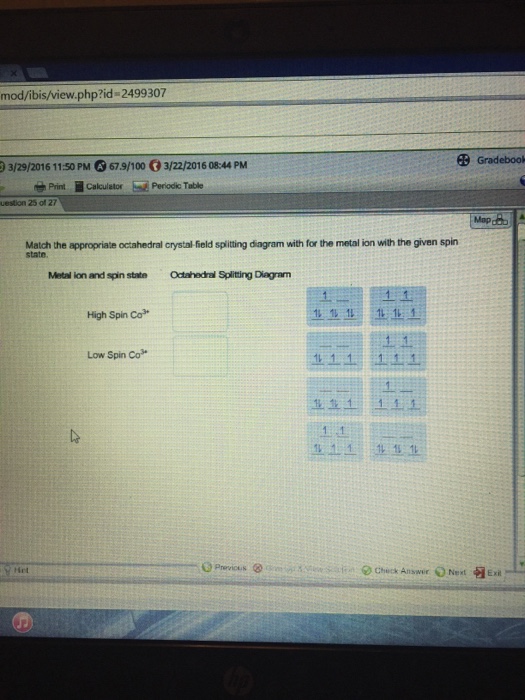
Match The Appropriate Octahedral Crystal Field Splitting Diagram
Match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. Given this is an octahedral complex your splitting diagram will have 2 degenerate states.
 Introduction To Inorganic Chemistry Coordination Chemistry And
Introduction To Inorganic Chemistry Coordination Chemistry And
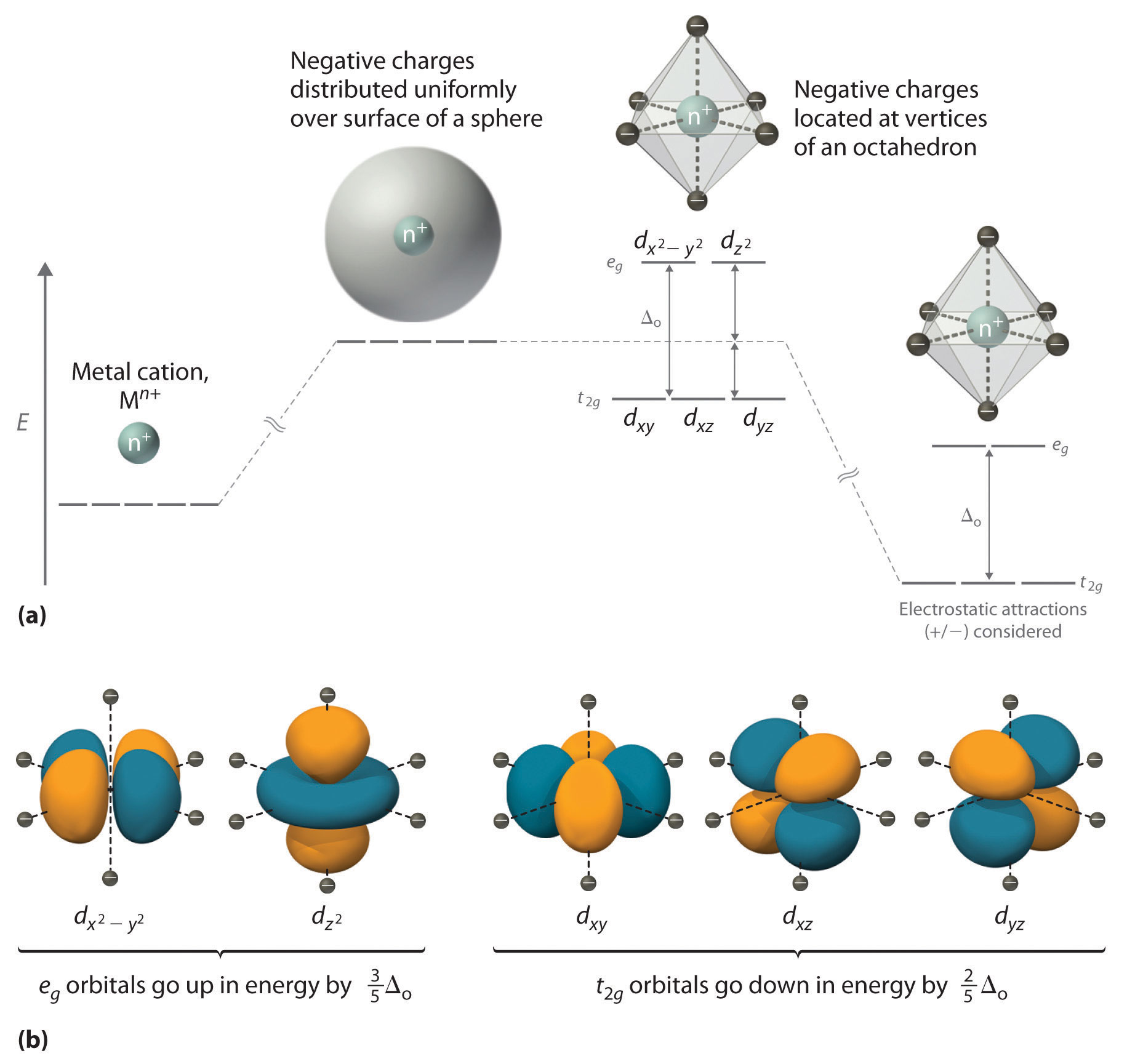
The dx2 y2 and dz2 orbitals on the metal ion at the center of the cube lie between the ligands and the dxy dxz and dyz orbitals point toward the ligands.

Match the appropriate octahedral crystal field splitting diagram. As a result the splitting observed in a tetrahedral crystal field is the opposite of the splitting in an octahedral complex. Solved match the appropriate octahedral crystal field spl match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion crystal field theory really need ur help what is the splitting energy of this crystal field theory really given this is an octahedral plex your splitting diagram will have. Metal ion and spin state octahedral splitting diagram show transcribed image text metal ion and spin state octahedral splitting diagram.
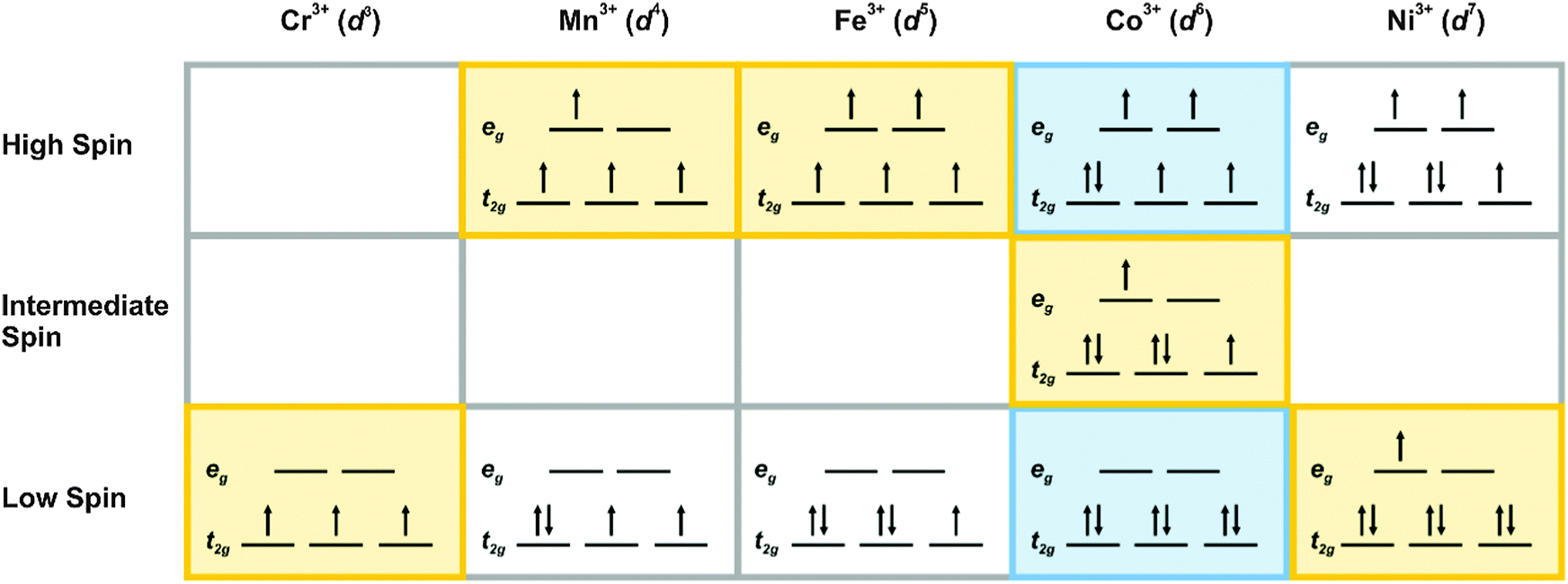
A left handed propeller will pull the stern to starboard right when in reverse. Boats and propellersif you have a single engine inboard installation the stern will pull to port left when you go into reverse if you have a righthanded propeller. This low spin state therefore does not follow hunds rule.
Cobalt 3 has 6 d electrons cobalt normally has 9 valence electrons but youve lost 3. High spin cr2 low spin fe3 please give the octahedral splitting diagrams for each ion. Consider ions to be from first row transition metals.
Which of the following are true for the crystal field model of an octahedral complex ion. Conversely the dconversely the dx2 y22 and the dxy orbitals increase in energy the splitting orbitals increase in energy. The splitting diagram for square planar complexes is more complex than for octahedral and tetrahedral complexes and is shown below with the relative energies of each orbital.
You have 6 br ions and your co complex is 3 that means youre dealing with co 3 as your metal center. Question match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. Match the appropriate octahedral crystal field splitting diagram.
A none of the 3d orbitals point directly at ligands b t2 orbitals are more stable than e orbitals c a small crystal field splitting energy results in a paramagnetic complex d the low spin case gives maximum unpaired electrons e for a given ligand. The octahedral ion feno 2 6 3 which has 5 d electrons would have the octahedral splitting diagram shown at right with all five electrons in the t 2g level. Lecture 7 crystal field theory for octahedral complexes.
Match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion.
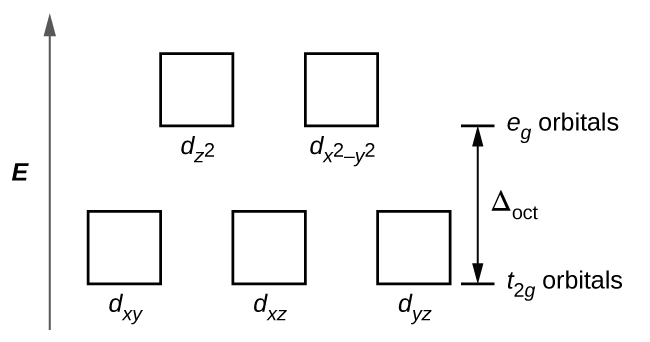 19 3 Spectroscopic And Magnetic Properties Of Coordination Compounds
19 3 Spectroscopic And Magnetic Properties Of Coordination Compounds
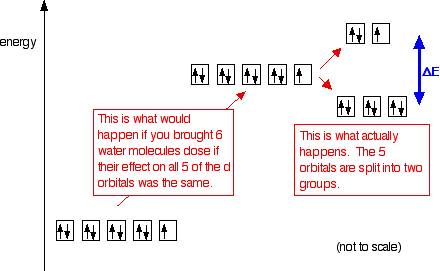 Complex Ions More About D Orbitals
Complex Ions More About D Orbitals
 Paramagnetic Resonance Of High Spin Co Ii In Biologically Relevant
Paramagnetic Resonance Of High Spin Co Ii In Biologically Relevant
 Magnetochemistry An Open Access Journal From Mdpi
Magnetochemistry An Open Access Journal From Mdpi
Universite De Geneve Groupe Du Professeur Andreas Hauser
Chapter 11 Coordination Chemistry Iii Electronic Spectra
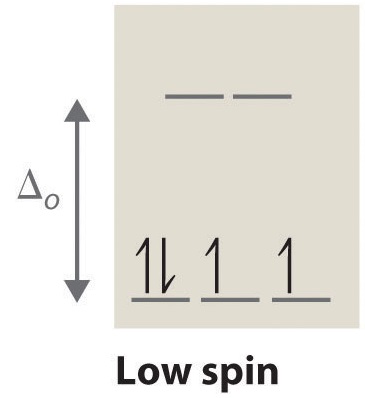 High Spin And Low Spin Complexes Chemistry Libretexts
High Spin And Low Spin Complexes Chemistry Libretexts
Ii Nuclear Spin Relaxation Studies Of Bromine In
 High Spin And Low Spin Complexes Chemistry Libretexts
High Spin And Low Spin Complexes Chemistry Libretexts
Topic 6 Coordination Compounds Coordination Chemistry
 Rare Earth Based Nanostructured Materials Synthesis
Rare Earth Based Nanostructured Materials Synthesis
 Magnetostructural Relations From A Combined Ab Initio And Ligand
Magnetostructural Relations From A Combined Ab Initio And Ligand
 Mossbauer And Electron Paramagnetic Resonance Studies Of
Mossbauer And Electron Paramagnetic Resonance Studies Of
Spectroscopic Characteristics Of Synthetic Olivine An Integrated
 Toward The Rational Design Of Non Precious Transition Metal Oxides
Toward The Rational Design Of Non Precious Transition Metal Oxides
 Tailoring Of The Trap Distribution And Crystal Field In Cr3 Doped
Tailoring Of The Trap Distribution And Crystal Field In Cr3 Doped


0 Response to "Match The Appropriate Octahedral Crystal Field Splitting Diagram"
Post a Comment