Which Electrons In This Diagram Contribute To The Stability Of The He2 Ion
F22 is not stable. One electron in the si.
 Molecular Orbital Mo Diagram Of H2 Youtube
Molecular Orbital Mo Diagram Of H2 Youtube
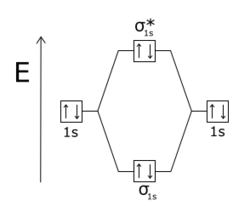
The two electrons in the sigma 1s mo.
Which electrons in this diagram contribute to the stability of the he2 ion. He h forms a very weak bond. 1 answer to which electrons in this diagram contribute to the stability of the ion. B the shapes of the molecular orbitals are obtained by squaring the wave functions for mo1 and mo2.
Which electrons in this diagram contribute to the stability of the ion. Which electrons in this diagram contribute to the stability of the ion. A the molecular orbital energy level diagram for the h2 molecule.
Please note the diagram is for he2 but the he h is very similar eg. The two electrons in the sigma 1s mo. If you add in two electrons they both go into the sigma anti bonding orbital and as a result the net bond order is zero.
The he 2 ion has a total of three electrons. So for h2 the two atoms share the two electrons in a covalent bond which gives it some stability. One electron in the sigma 1s mo and another one in the sigma 1s mo.
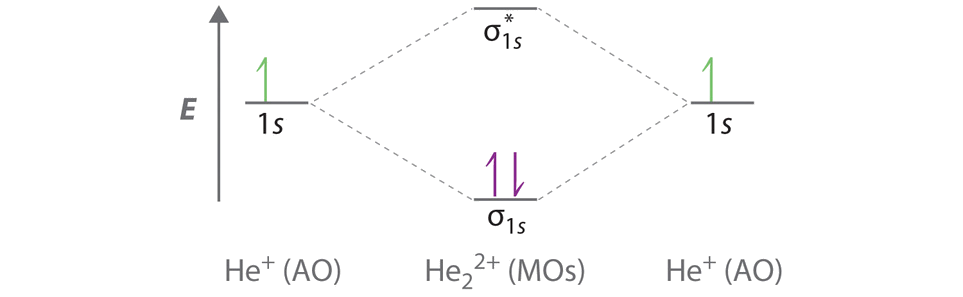
Two are placed in the bonding orbital the third in the antibonding orbital. Solve the energy level diagram for the he 2 ion is shown in figure 934. Thus the bond order is because the bond order is greater than 0 the he 2 molecular ion is predicted to be stable relative to the separated he and he.
Two are placed in the bonding orbital and the third in the antibonding orbital. The electron in the sigma 1s mo. If there are the same amount of protons and electrons in an atom then it is neutral but if it has more of one or the other then it has a positive or negative ion.
He2 would have a bond order of zero but he2 would have a bond order of 05 2 e in the bonding orbital and 1 electron in the anti bonding orbital. This mix to form a sigma orbital from h1sli2s a sigma orbital and h1s li2s and a non bonding orbital from li1s. He exits as a single atom molecule not as he2 with a complete 2s2 structure.
Problem energy level diagram for the he 2 ion. If the bond order is greater than 0 we expect a bond to exist and the ion is stable. Energy level diagram for the he2 ionwhich electrons in this diagram contribute to the stability of the he2 ion.
Li has 1s 2s while h has 1s. This ion has three electrons. Energy level diagram for thehe2 ion.
H2 also has two protons in two nuclei that affect the bonding by contributing two centers of positive charge. The electron in the sigma 1s mo. One electron in the sigma 1s mo and another one in the sigma 1s mo.
Because the energy of the two electrons is lower than the energy of the individual atoms the molecule is stable. The valence electrons of he are in the 1s orbital and the 1s orbitals combine to give an mo diagram like that for h 2 or he 2 figure 933.
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 Antibonding Molecular Orbital Wikipedia
Antibonding Molecular Orbital Wikipedia
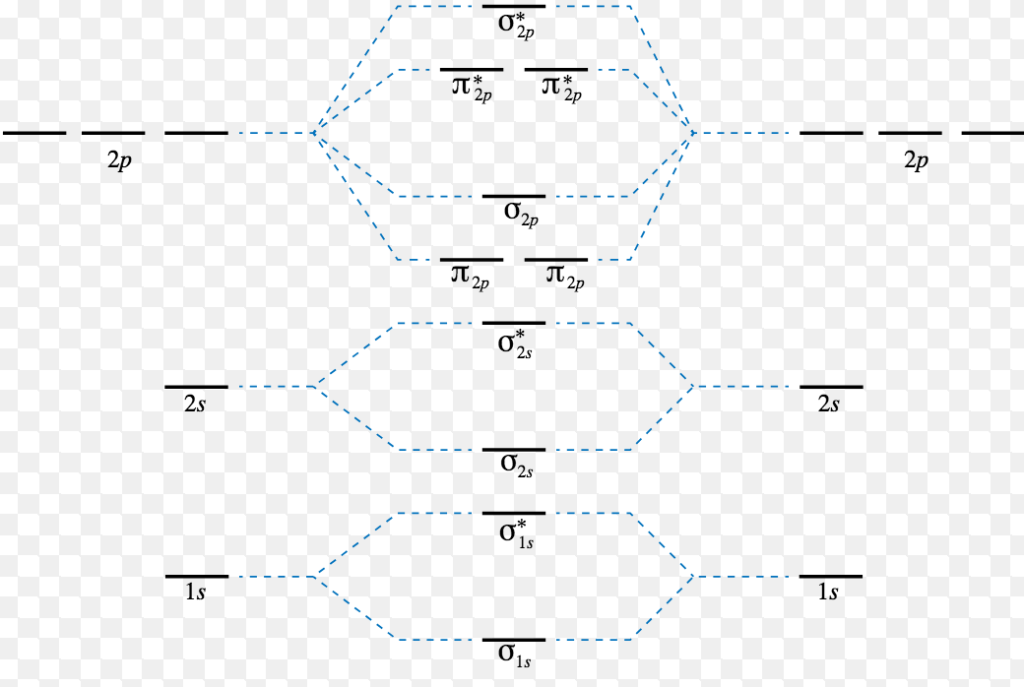
Chapter 2 Molecular Structure And Bonding
 Energy Level Diagram For Molecular Orbitals Chemical Bonding And
Energy Level Diagram For Molecular Orbitals Chemical Bonding And
 Ion Efficiency Curves For He And He Formed Via Electron
Ion Efficiency Curves For He And He Formed Via Electron
 Energy Level Diagram For The He2 Ion W Clutch Prep
Energy Level Diagram For The He2 Ion W Clutch Prep
Which One Is More Stable H2 Or H2 Quora
Organic Chemistry I New Intro To Molecular Orbital Mo Theory
 Chemical Bonding Molecular Orbitals Of H2 And He2 Britannica Com
Chemical Bonding Molecular Orbitals Of H2 And He2 Britannica Com
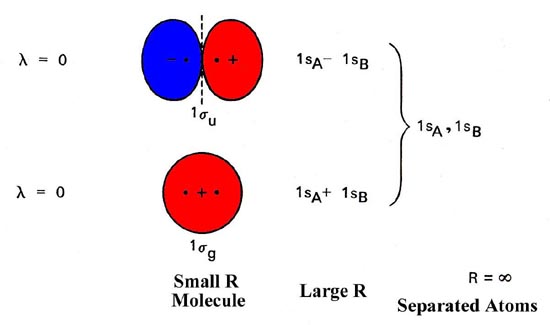 Molecular Orbitals Molecular Orbitals For Homonuclear Diatomics
Molecular Orbitals Molecular Orbitals For Homonuclear Diatomics

 8 5 Molecular Orbital Theory Chemistry
8 5 Molecular Orbital Theory Chemistry
 Imaging The He2 Quantum Halo State Using A Free Electron Laser Pnas
Imaging The He2 Quantum Halo State Using A Free Electron Laser Pnas
 9 7 Molecular Orbitals Chemistry Libretexts
9 7 Molecular Orbitals Chemistry Libretexts
 Electron Attachment And Electron Ionization Of Formic Acid Clusters
Electron Attachment And Electron Ionization Of Formic Acid Clusters

 How Do I Calculate The Bond Order For H2 And H2 Socratic
How Do I Calculate The Bond Order For H2 And H2 Socratic
 Wave Particle Energy Exchange Directly Observed In A Kinetic Alfven
Wave Particle Energy Exchange Directly Observed In A Kinetic Alfven
Which Is More Stable He2 Or H2 And Why Quora
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
0 Response to "Which Electrons In This Diagram Contribute To The Stability Of The He2 Ion"
Post a Comment