Which Of The Following Diagrams Represent A Weak Acid Dissolved In Water
A h2o b kcl c hno3 d hc2h3o2 e c12h22o11. Ios android web.
 Openstax General Chemistry Ch 14 Acid Base Equilibria Top Hat
Openstax General Chemistry Ch 14 Acid Base Equilibria Top Hat
Hc2h3o2aq h2ol h3oaq c2h3o2 aq or hc2h3o2aq haq c2h3o2 aq 18 10 5.

Which of the following diagrams represent a weak acid dissolved in water. Which represents a weak acid. 3identify each of the following substances as a strong electrolyte weak electrolyte or nonelectrolyte. Which represents a weak acid.
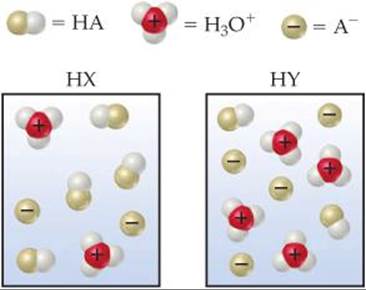
Water molecules are omitted for clarity strong acid a b c d weak acida b c dvery weak acid a b c d. Which represents a weak acid. Acids and bases that are strong electrolytes completely ionized in solution are called strong acids and strong bases.
The diagram best represent the hydration is c. 1622 label each of the following as being a strong acid a weak acid or a species with negligible acidity. Strong and weak acids and bases.
Water molecules are omitted for clarity. 712 which of the following diagrams best represents a strong acid such as hcl dissolved in water. Which of the following diagrams best represents a strong acid such as hcl dissolved in water.
Only a fraction of the nh3 reacts with h2o. The hydrated proton is shown as a hydronium ion. Nh3 is a weak electrolyte.
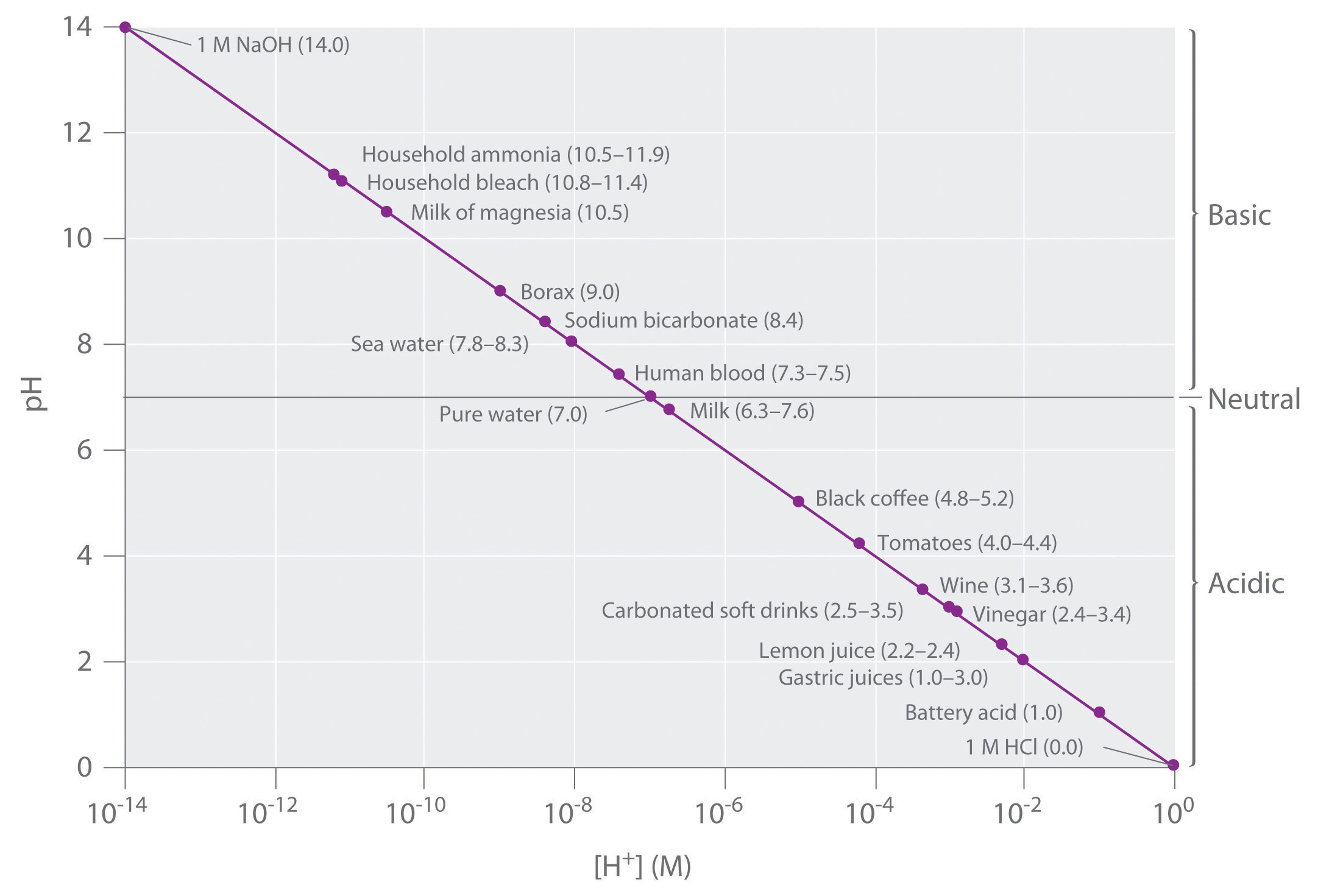
Which represents a very weak acid. 000010m the percent ionization of a weak acid increases as the initial concentration of the acid decreases ph values in order of ph increasing h₃o starting with the ph that corresponds to the lowest h₃o at the top of the list. With strong acids this is easy.
Which represents a very weak acid. Which represents a very weak acid. Water molecules are omitted for clarity chapter problem is solved.
Figure 46 an h2o molecule acts as a proton donor acid and nh3 as a proton acceptor base. The hydrated proton is shown as a hydronium ion. Water molecules are omitted for clarity a b c d.
Which of the following diagrams best represents a strong acid such as hcl dissolved in water. Which of the following diagrams best represents a strong acid such as hcl dissolved in water. Which represents a very weak acid.
For example acetic acid is a weak acid because when it is added to water it reacts with the water in a reversible fashion to form hydronium and acetate ions. Because nacl ions are separated and h positive inos is attracted to cl which are negative. Which represents a weak acid.
That means that if the concentration of the acid is 01 mol dm 3 then the concentration of hydrogen ions is also 01 mol dm 3. The hydrated proton is shown as a hydronium ion. The hydrated proton is shown as a hydronium ion.
In each case write the formula of its conjugate base and indicate whether the conjugate base is a strong base a weak base or a species with negligible basicity. Hydrochloric acid is a strong acid virtually 100 ionised. Each mole of hcl reacts with the water to give 1 mole of hydrogen ions and 1 mole of chloride ions.
A hcooh b h 2 c ch 4 d hf e nh 4.
11 3 Finding The Ph Of Weak Acids Bases And Salts Chemistry
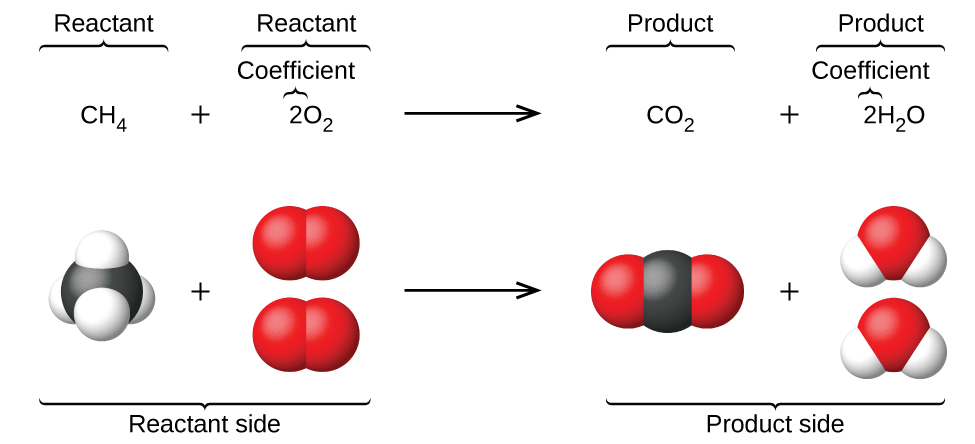 4 1 Writing And Balancing Chemical Equations Chemistry
4 1 Writing And Balancing Chemical Equations Chemistry
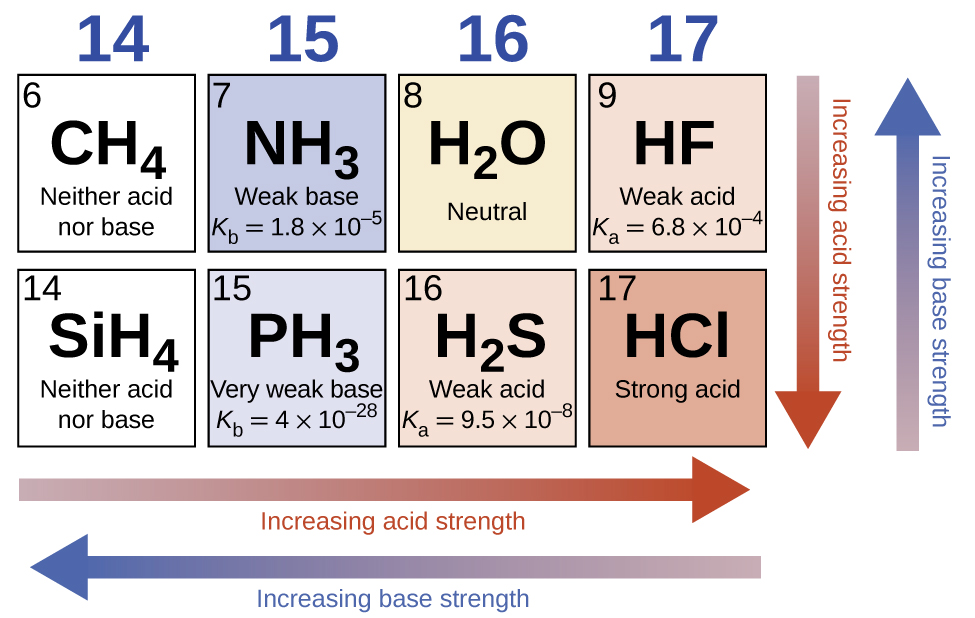 14 3 Relative Strengths Of Acids And Bases Chemistry
14 3 Relative Strengths Of Acids And Bases Chemistry
 Hydrogen Chloride Vs Hydrochloric Acid Video Lesson Transcript
Hydrogen Chloride Vs Hydrochloric Acid Video Lesson Transcript
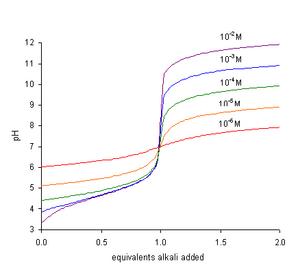 Neutralization Chemistry Wikipedia
Neutralization Chemistry Wikipedia
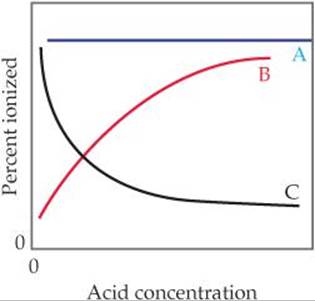 Exercises Acid Base Equilibria Chemistry The Central Science
Exercises Acid Base Equilibria Chemistry The Central Science
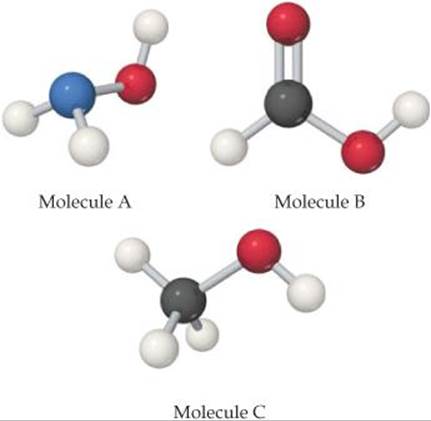 Exercises Acid Base Equilibria Chemistry The Central Science
Exercises Acid Base Equilibria Chemistry The Central Science
 Chapter Ppt Video Online Download
Chapter Ppt Video Online Download
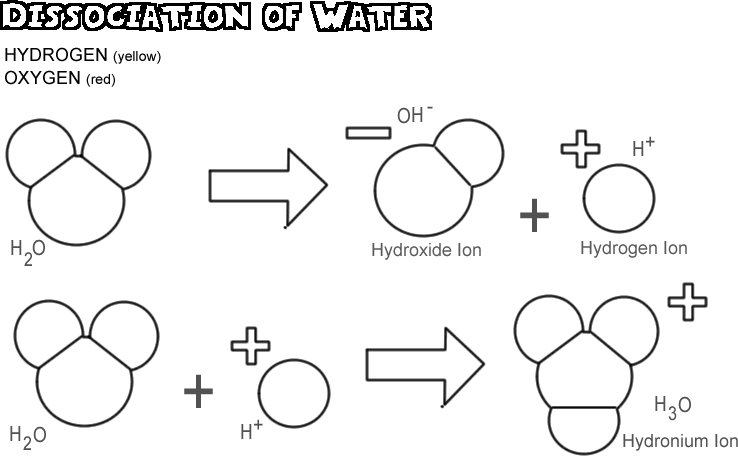 Acids Bases And The Dissociation Of Water
Acids Bases And The Dissociation Of Water

Maintaining Cellular Conditions Ph And Buffers
Chapter 16 Acid Base Equilibria
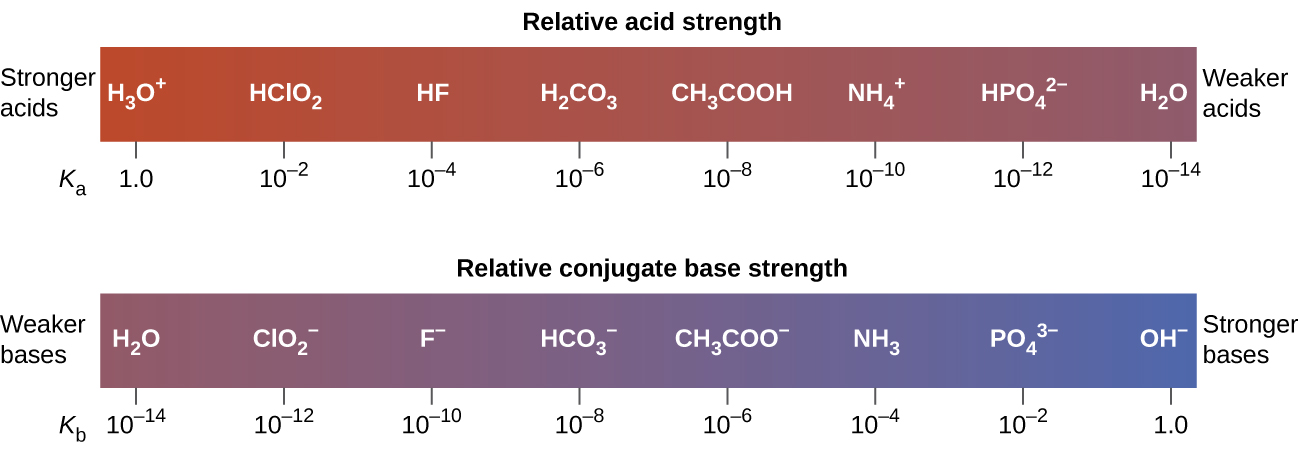 14 3 Relative Strengths Of Acids And Bases Chemistry
14 3 Relative Strengths Of Acids And Bases Chemistry
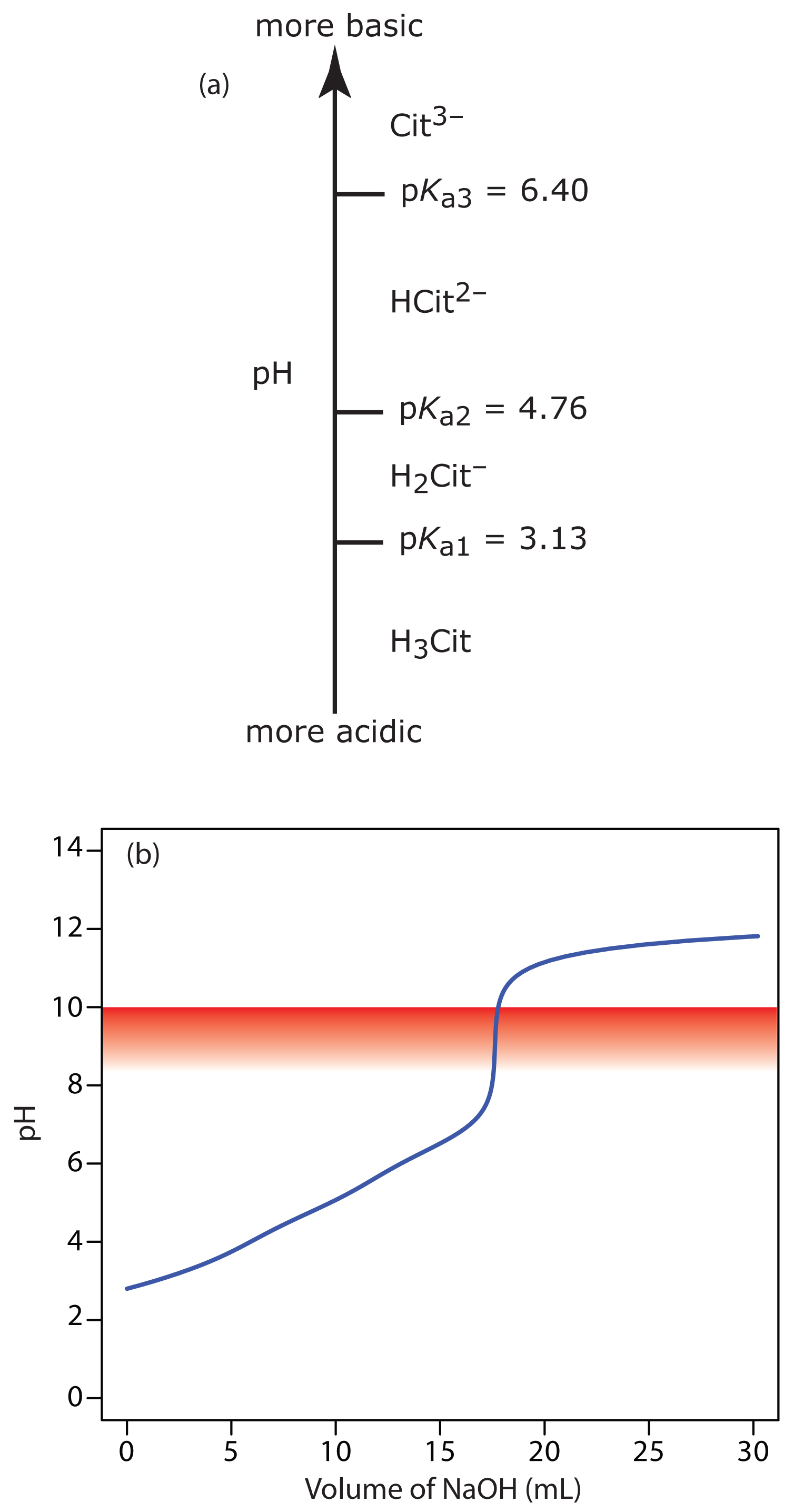 9 2 Acid Base Titrations Chemistry Libretexts
9 2 Acid Base Titrations Chemistry Libretexts
 Exercises Acid Base Equilibria Chemistry The Central Science
Exercises Acid Base Equilibria Chemistry The Central Science
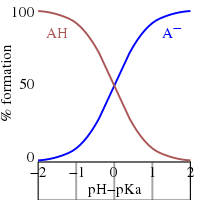 Acid Dissociation Constant Wikipedia
Acid Dissociation Constant Wikipedia
Definitions Of Acids And Bases And The Role Of Water
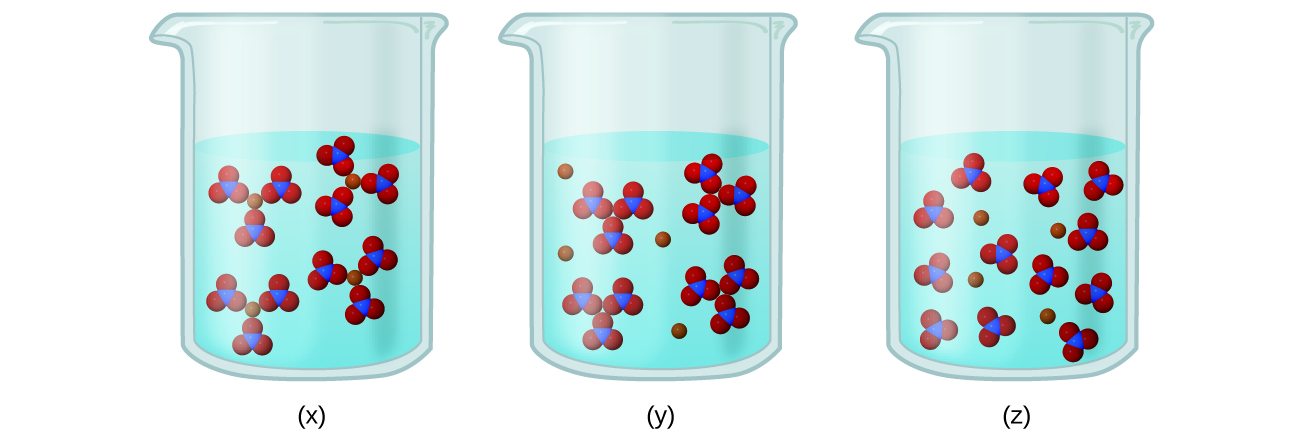
 4 3 Acid Base Reactions Chemistry Libretexts
4 3 Acid Base Reactions Chemistry Libretexts
0 Response to "Which Of The Following Diagrams Represent A Weak Acid Dissolved In Water"
Post a Comment