Which Statement Is True Of The Atom Shown In The Diagram
The diagram represents a portion of the esophagus. The second shell should have 8 electrons.
 Matter Is Anything That Has Mass Weight And Occupies Space Ppt
Matter Is Anything That Has Mass Weight And Occupies Space Ppt
The only sp2 hybridized atom is c 2 b.
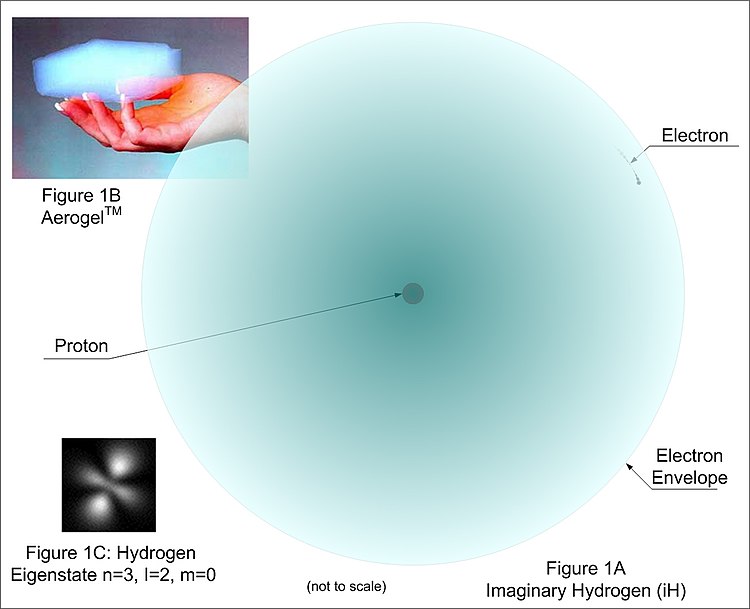
Which statement is true of the atom shown in the diagram. All atoms of the same element are identical. Mass number 16 atomic number 7. The second shell cant have 4 electrons.
The first shell must fill before the second shell can have electrons. Radioactive decay is likely to occur when. No shell can hold more than 2 electrons.
An atom has 8 protons 8 neutrons and 8 electrons. The chemical basis of life. Another isotope of the same element might have.
All of the above. An atom has too many electrons. N 4 is sp3 hybridized.
Someone should tell dr. 2 it must occur prior the mechanical digestion of food in the oral cavity. The only sp3 hybridized atoms are c 1 c 3 and c 5 c.
The valence shell has higher energy than other occupied shells. 3 it emulsifies fats for hydrolysis in the small intestine 4 it increases water absorption by the esophagus this is the picture. B electrons must lose energy to move from the first to the second shell.
C 2 and o 6 are sp2 hybridized. The picture shows one big red one in the middle with one blue ring on the outside that had 2 smaller red ones and another blue ring outside of the first blue ring that had 8 smaller red ones. Designate the atom shown in question 6 in the form name of element mass.
The atom needs 3 more electrons to fill the valence shell. Which statement is true of the energy levels of electrons in shells. Circle the letter of each statement that is true about the average atomic mass.
Which is a correct statement about the process shown in the diagram. When elements react their atoms combine in simple whole number ratios. Which of the following is true about the atom shown choose two that apply.
Jones says an atom has 3 electrons in the first shell and four electrons in the second shell. Chapters 2 and 3 study guide chapters 2 and 3 the mass. Two electrons fill the first shell and 5 go into the second valence shell.
All of the above. None of the above. 5 atomic structure and the periodic table atoms they did not explain chemical behavior and they lacked experimental support.
C 2 o 6 and n 4 are all sp2 hybridized d. By making two covalent bonds an o atom with 8 protons fills its valence shell. Around the 1870s this scientist studied mysterious rays in sealed glass tubes.
1 it transports nutrients within the digestive tract. Which of the following statements is true regarding the structure shown. The neutral atom has 7 electrons.
Which of the following statements is true regarding the structure shown. All matter consists of tiny particles called atoms. All the electrons in an atom have similar amounts of energy.
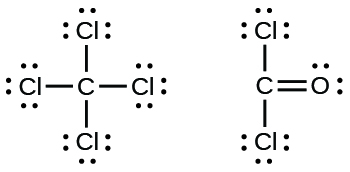 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
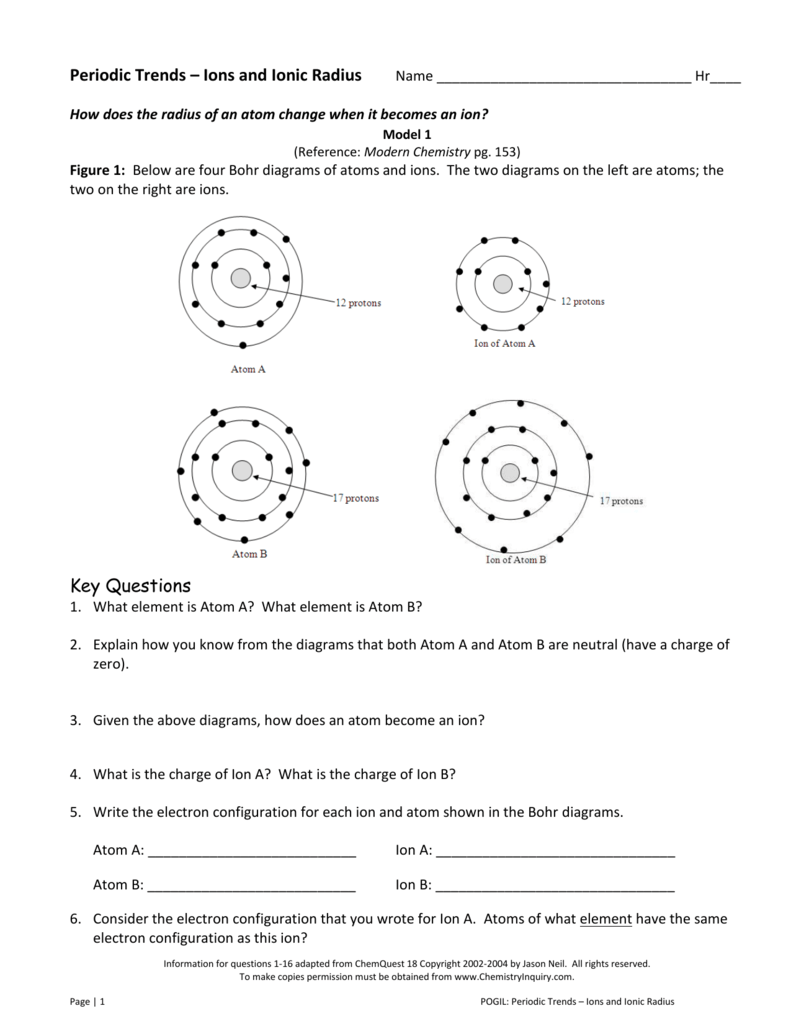 Periodic Trends Ions And Ionic Radius Name
Periodic Trends Ions And Ionic Radius Name
 Molecule Definition Examples Structures Facts Britannica Com
Molecule Definition Examples Structures Facts Britannica Com
 Collective Autoionization In Multiply Excited Systems A Novel
Collective Autoionization In Multiply Excited Systems A Novel
 Parallel Efficiency Versus Atoms Per Node For Benchmark Simulations
Parallel Efficiency Versus Atoms Per Node For Benchmark Simulations
 Structure Of Microtubules A Atomic Structure Of The Ab Tubulin
Structure Of Microtubules A Atomic Structure Of The Ab Tubulin
![]() Methamphetamine Crystal Meth Drug Molecule Flat Icon Style
Methamphetamine Crystal Meth Drug Molecule Flat Icon Style
 Chemistry I Atoms And Molecules
Chemistry I Atoms And Molecules
 Thomson Atomic Model Description Image Britannica Com
Thomson Atomic Model Description Image Britannica Com
:quality(75)/curiosity-data.s3.amazonaws.com/images/content/landscape/standard/57e62fce-1abc-4cb2-c1a0-8ede2b31ec64.png) The Atom Diagram Isn T What An Atom Looks Like
The Atom Diagram Isn T What An Atom Looks Like
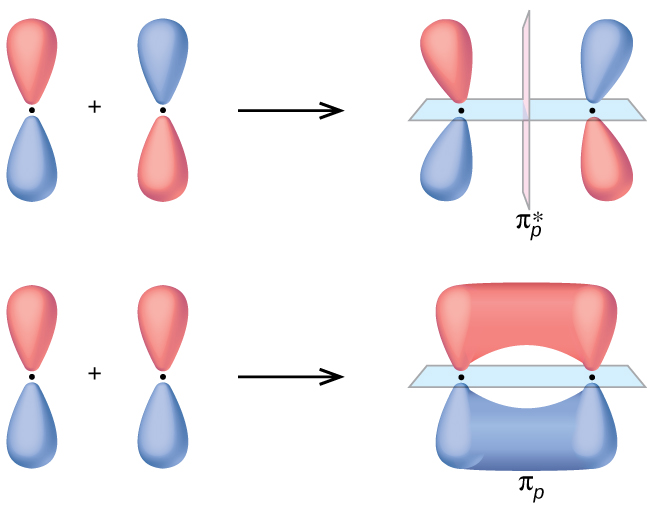 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 What Are The Parts Of An Atom Universe Today
What Are The Parts Of An Atom Universe Today
 A New Model Of The Atom Wikibooks Open Books For An Open World
A New Model Of The Atom Wikibooks Open Books For An Open World
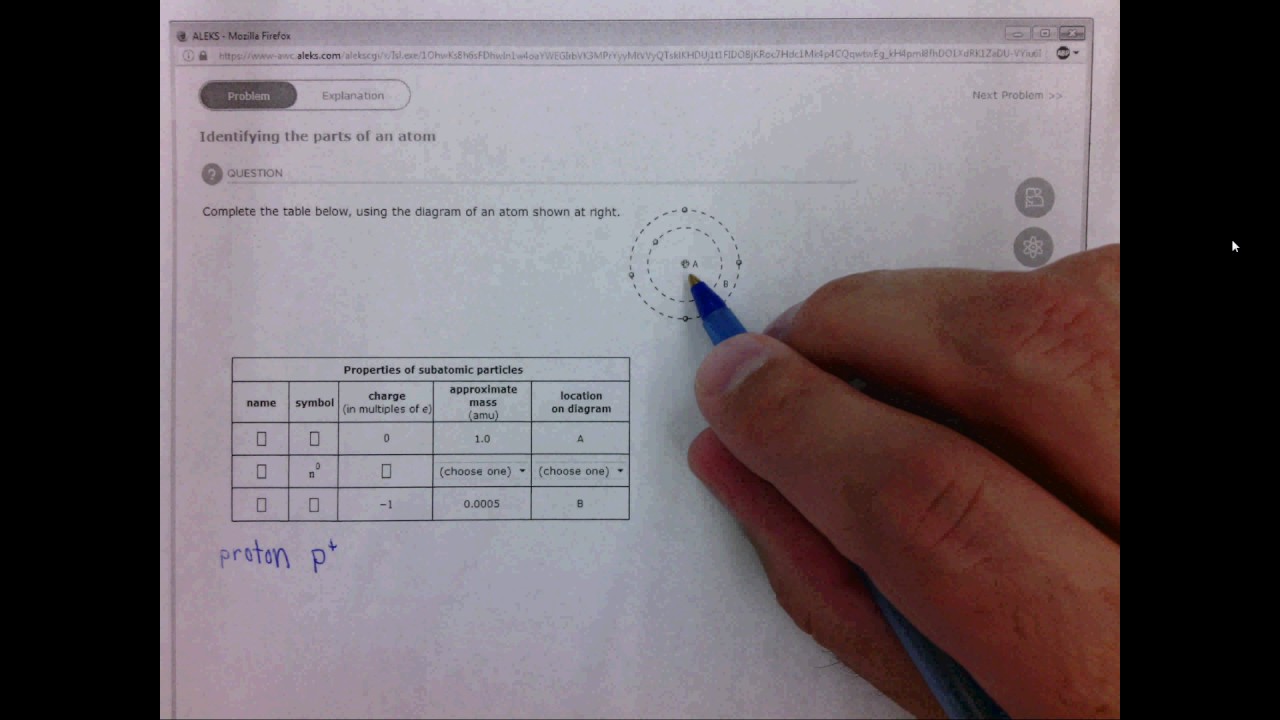 4 1a Identifying The Parts Of An Atom Youtube
4 1a Identifying The Parts Of An Atom Youtube
:max_bytes(150000):strip_icc()/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg) Basic Model Of The Atom Atomic Theory
Basic Model Of The Atom Atomic Theory
:quality(75)/curiosity-data.s3.amazonaws.com/images/content/thumbnail/standard/7745ec41-a92d-479f-c88b-df90a6518f5c.png) The Atom Diagram Isn T What An Atom Looks Like
The Atom Diagram Isn T What An Atom Looks Like
:max_bytes(150000):strip_icc()/Iron-58b602243df78cdcd83d3d5a.jpg) Atom Diagrams Electron Configurations Of The Elements
Atom Diagrams Electron Configurations Of The Elements
 Mastering Biology Answers Chemistry Review Atoms Molecules
Mastering Biology Answers Chemistry Review Atoms Molecules
 A New Model Of The Atom Wikibooks Open Books For An Open World
A New Model Of The Atom Wikibooks Open Books For An Open World


0 Response to "Which Statement Is True Of The Atom Shown In The Diagram"
Post a Comment