No Molecular Orbital Diagram
In chemistry a molecular orbital mo is a mathematical function describing the wave like behavior of an electron in a molecule. The bonding orbitals are slightly more concentrated on o.
 Pdf Lewis Acidity Of No And No2 As Measured By Their Affinity To
Pdf Lewis Acidity Of No And No2 As Measured By Their Affinity To
These are uvvisible infra red ir and nuclear magnetic resonance nmr spectroscopies.
No molecular orbital diagram. Now draw two more mo diagrams for no and no your no diagram will have one less valence electron and your no diagram will have one more valence electron. Molecular orbitals of no. An honors general chemistry computational lab as implemented using three dimensional modeling software journal of chemical education ruddick parrill and petersen 2012 89 11 pp 13581363 abstract.
The course introduces the three key spectroscopic methods used by chemists and biochemists to analyse the molecular and electronic structure of atoms and molecules. Step 1 of 5 molecular orbitals are formed by linear combination of atomic orbitals. Compare the bond orders in these two ions.
Computational study of the interaction between no no and no with h2o. In chemistry molecular orbital mo theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms but are treated as moving under the influence of the nuclei in the whole molecule. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular.
The bond order is 25 with one unpaired electron. O is more electronegative than n so its orbitals are slightly lower in energy and the bonding orbitals are slightly more concentrated on o. Note the odd electron is in a pi2p orbital.
Molecular orbital diagrams of diatomic molecules introduction. Introductory molecular orbital theory. Draw the molecular orbital diagrams for no and no.
No bond order 3 shortest bond 106 pm no bond order 25 intermediate 115 pm no bond order 2 longest bond 127 pm two electrons in antibonding orbitals. Molecular orbital diagram of no endorsed post by claudettecontr3i tue nov 15 2016 1000 pm i am not quiet sure but it seems that the book may have taken the electron off o because then it would have the same amount of electrons in the 2p orbital as nitrogen it looks neater. Atomic orbitals and molecular orbitals of a molecule can be shown in a molecular orbital diagram.
This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region.
 Basic Sites In Zeolites Followed By Ir Studies Of No Sciencedirect
Basic Sites In Zeolites Followed By Ir Studies Of No Sciencedirect

 Chemistry 481 01 Spring 2017 Instructor Dr Upali Siriwardane
Chemistry 481 01 Spring 2017 Instructor Dr Upali Siriwardane
 Write M O T Of No Calculate Its Bond Order Brainly In
Write M O T Of No Calculate Its Bond Order Brainly In
 What Is The Bond Order Of Co Quora
What Is The Bond Order Of Co Quora
Chem 2303 Supplementary Problems

 Solved The Diatomic Cyanide Ion Cn And Nitrosonium Ion No
Solved The Diatomic Cyanide Ion Cn And Nitrosonium Ion No
Molecular Orbital Treatment For Heteronuclear Diatomic Molecules
Chem 2303 Supplementary Problems
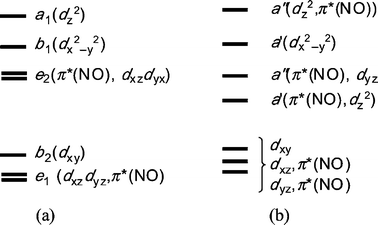 The Coordination Chemistry Of Nitrosyl In Cyanoferrates An Exhibit
The Coordination Chemistry Of Nitrosyl In Cyanoferrates An Exhibit
Answer On The Question 54656 Chemistry General Chemistry
 Mo Diagram Of No 7 2 Stromoeko De
Mo Diagram Of No 7 2 Stromoeko De
 Chapter 10 Molecular Geometry And Chemical Bonding Theory Ppt
Chapter 10 Molecular Geometry And Chemical Bonding Theory Ppt
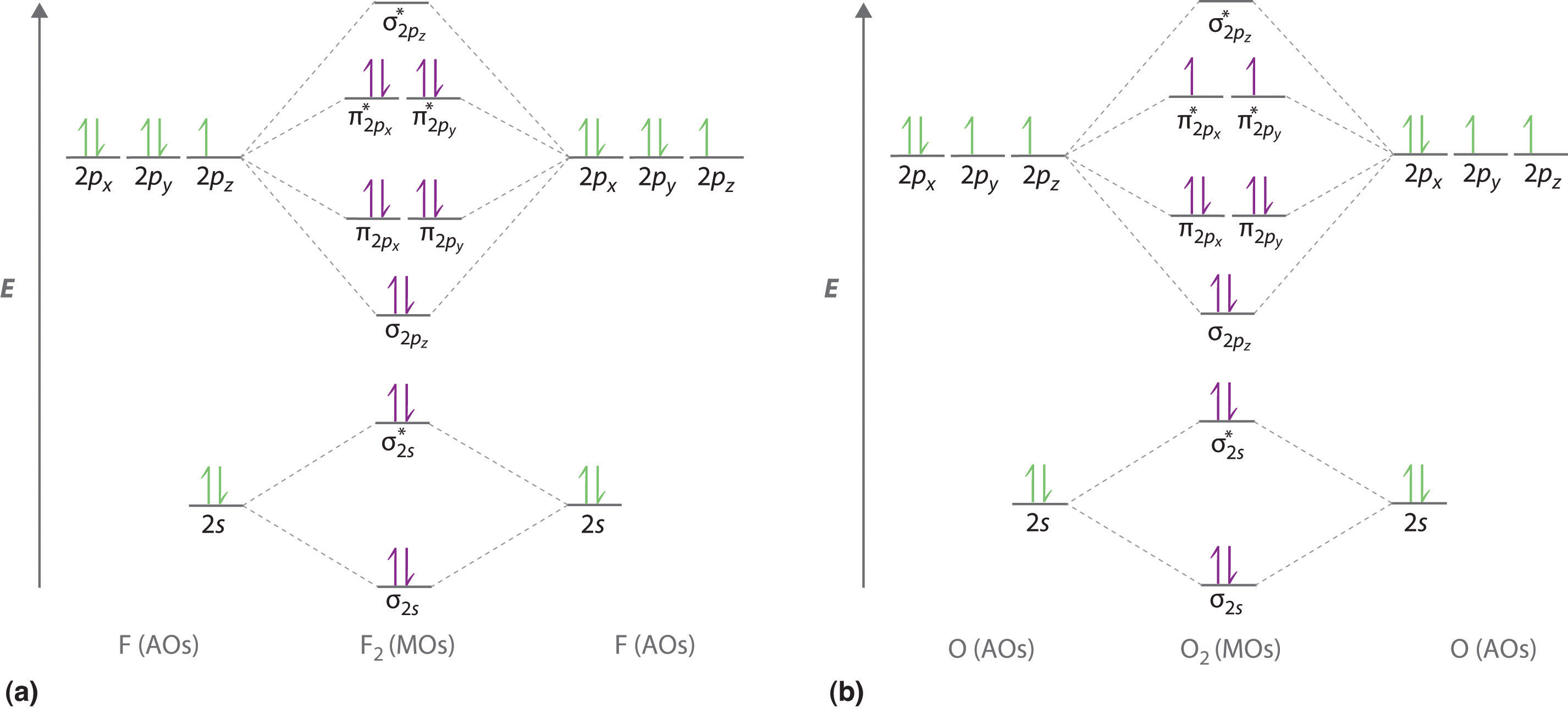 Chapter 6 5 Delocalized Bonding And Molecular Orbitals Chemistry
Chapter 6 5 Delocalized Bonding And Molecular Orbitals Chemistry
 By Writing Molecular Orbital Configuration For No Co O2 Molecules
By Writing Molecular Orbital Configuration For No Co O2 Molecules

0 Response to "No Molecular Orbital Diagram"
Post a Comment