The Orbital Diagram For A Ground State Oxygen Atom Is
Electrons in an orbital with l 3 are in a a. So since you have 4 electrons in the p orbital 2 of them will get there own orbital and 2 will have to share an orbital but they will spin in opposite directions.
 Molecular Orbital Diagram For A Simple Pi Bond Bonding And
Molecular Orbital Diagram For A Simple Pi Bond Bonding And
The ground state is 1s2 2s2 2p2.
The orbital diagram for a ground state oxygen atom is. C has two unpaired electrons in its ground state. So as you may know in the p subshell there are 3 orbitals x y z that can hold 2 electrons each. The orbital diagram for a ground state oxygen atom is a a b b c c d d e e which element has the following ground state electron configuration.
To provide services that meet the expectations of a client requires an investment in experts in research customer support every relevant department and more. This subshell is full. Of these colors has the least energy.
A possible set of quantum numbers for the last electron added to complete an atom of germanium in its ground state is a. The 3s subshell contains one orbital ml0 which holds two spin paired electrons. The colors of the visible spectrum are red orange yellow green blue and violet.
Which ground state atom has an electron configuration described by the following orbital diagram. The ground state electron configuration of p is ne3s23p3. In the explanation below i show a common means of diagramming this.
The orbital diagram for a ground state oxygen atom is. Make your school life easier by placing an order with us. Of these colors has the most energy.
1s22s22p63s2 a na b mg c ai d si e ne a ground state atom of manganese has unpaired electrons and is. Question description the orbital diagram for a ground state oxygen atom is see more best custom paper writing platform. Which of these choices is the electron configuration of an excited state of an oxygen atom.
The 4p subshell contains three orbitals ml 1 0 1. 1s2 2s2 2p3 3s1. The colors of the visible spectrum are red orange yellow green blue and violet.
Because oxygen has 8 electrons its configuration is 1s2 2s2 2p4.
 Chapter7 Seat Work Answers Docx General Chemistry Sci 154 Seat
Chapter7 Seat Work Answers Docx General Chemistry Sci 154 Seat
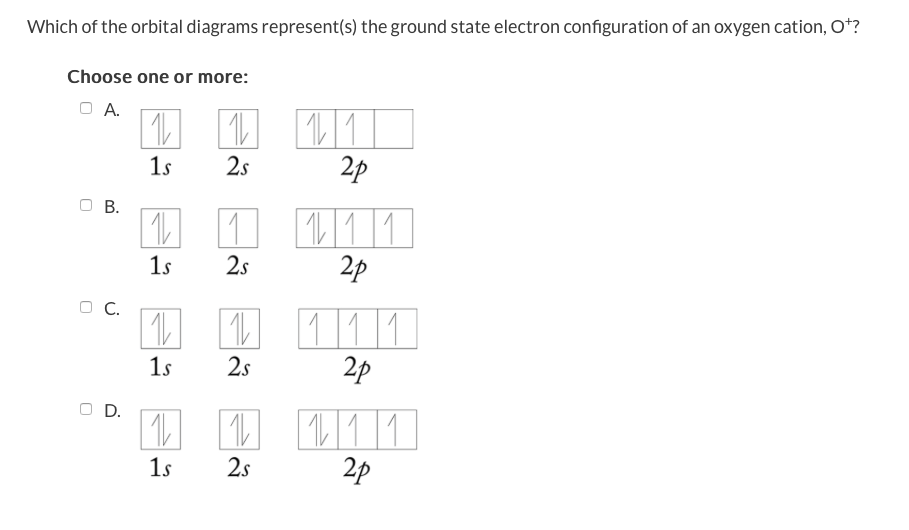 Solved 13 Question 3 Points A See Page 367 Orbital Diagr
Solved 13 Question 3 Points A See Page 367 Orbital Diagr
 Ground State Electron Configuration Definition Example Video
Ground State Electron Configuration Definition Example Video
 Savvy Chemist Carbonyl Compounds 1 Structure Of The Carbonyl Group
Savvy Chemist Carbonyl Compounds 1 Structure Of The Carbonyl Group
 6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
Chem 1411 Study Guide For Test 3 Chapters 7 8 Pages 1 8 Text
 What Is The Hybridization Of Hybrid Orbitals Of H20 Molecule Quora
What Is The Hybridization Of Hybrid Orbitals Of H20 Molecule Quora
 Five Reaction Chemistries Chemogenesis
Five Reaction Chemistries Chemogenesis

 Figure 1 From Generalized Valence Bond Description Of Chalcogen
Figure 1 From Generalized Valence Bond Description Of Chalcogen
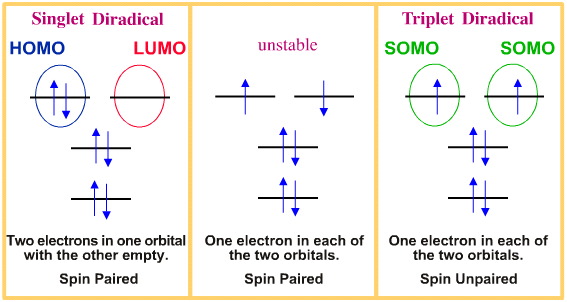 Diradical Chemistry Chemogenesis
Diradical Chemistry Chemogenesis
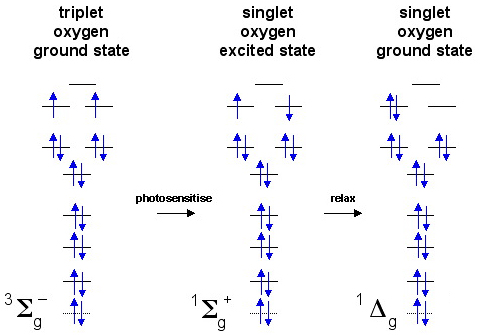
Quantum Mechanics Why Is Oxygen In A Triplet State And What Are
 Dublin Schools Lesson Orbital Diagrams And Electron Configurations
Dublin Schools Lesson Orbital Diagrams And Electron Configurations
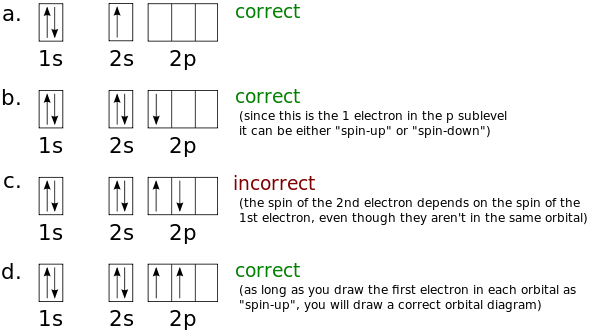 High School Chemistry Orbital Configurations Wikibooks Open Books
High School Chemistry Orbital Configurations Wikibooks Open Books
 Electron Diagrams And Excited State Youtube
Electron Diagrams And Excited State Youtube
 The Orbital Diagram For A Ground State Oxygen Atom Is A Row 1 B Row
The Orbital Diagram For A Ground State Oxygen Atom Is A Row 1 B Row

 Electron Configurations The Periodic Table
Electron Configurations The Periodic Table
 Chapter 10 Chemical Bonding Ii Molecular Geometry And Hybridization
Chapter 10 Chemical Bonding Ii Molecular Geometry And Hybridization
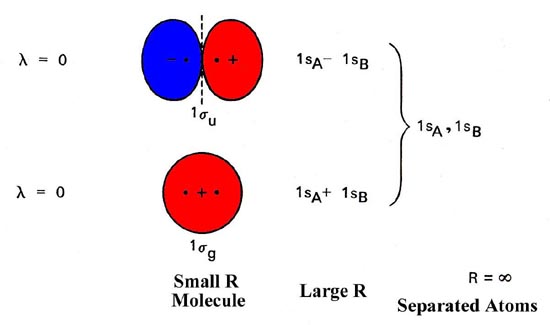 Molecular Orbitals Molecular Orbitals For Homonuclear Diatomics
Molecular Orbitals Molecular Orbitals For Homonuclear Diatomics
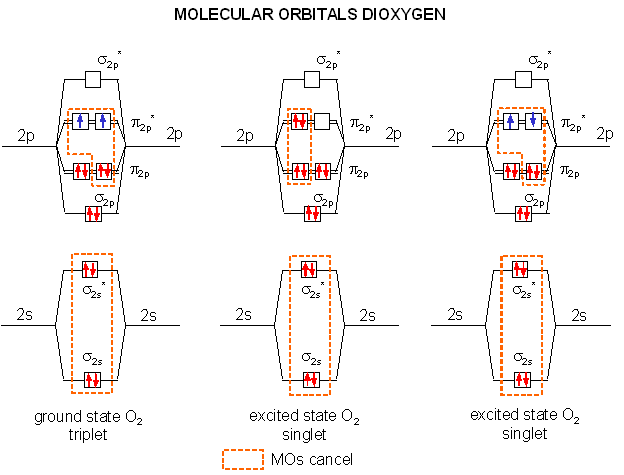
0 Response to "The Orbital Diagram For A Ground State Oxygen Atom Is"
Post a Comment