Atomic Orbital Diagram For Chlorine
Electron configurations and orbital diagrams key draw orbital diagrams for the following elements. How many electrons does a fe atom have in its 3.
 A P Dos Of Lead And Chlorine Atoms In The Aqpbcl 4 Compound And The
A P Dos Of Lead And Chlorine Atoms In The Aqpbcl 4 Compound And The
Since the 3s if now full well move to the 3p where well place the remaining five electrons.

Atomic orbital diagram for chlorine. An orbital diagram is a sketch which shows electron population in atomic orbitals with the electrons spin indicated by up and down arrows. Chlorine cl has an atomic mass of 17. In a chlorine atom which subshells contain valence electrons.
The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Two atomic orbitals in phase create a larger electron density which leads to the σ orbital. The superposition of the two 1s atomic orbitals leads to the formation of the σ and σ molecular orbitals.
Create the atomic orbital diagram for nitrogen. Phosphorus 1s 2s 2p 3s 3p 4s 3d 4p 2. Create the atomic orbital diagram for chlorine.
Elements are show from atomic number 1 hydrogen up to 94 plutonium. On the right hand side are four pull down menus from which you can choose an orbital to display. Therefore the chlorine electron configuration will be 1s22s22p63s23p5.
Show transcribed image text create the atomic orbital diagram for chlorine. For each atom diagram the element symbol is listed in the nucleus. Construct the orbital diagram of each atom or ion.
In atomic theory and quantum mechanics an atomic orbital is a mathematical function that describes the wave like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atoms nucleus. Draw the atomic orbital diagram for chlorine.
The green dot represents the location of a chlorine nucleus significantly enlarged so that you can see it. The p orbital can hold up to six electrons. The electronic configuration of a ground state chlorine is ne3s23p5 1s22s22p63s23p5.
Find out about its chemical and physical properties states energy electrons oxidation and more. If the two 1s orbitals are not in phase a node between them causes a jump in energy the σ orbital. Draw the atomic orbital diagram for chlorine.
The electron shells are shown moving outward from the nucleus. Well put six in the 2p orbital and then put the next two electrons in the 3s.
 Why Is Bcl3 An Electron Deficient Compound And Why Does It Have A
Why Is Bcl3 An Electron Deficient Compound And Why Does It Have A
Chem4kids Com Nitrogen Orbital And Bonding Info
Electron Configuration Of Chlorine Chlorine Table Of Elements By
What Is The Ground State Configuration Of Chlorine Socratic
 Electron Configuration Boundless Chemistry
Electron Configuration Boundless Chemistry
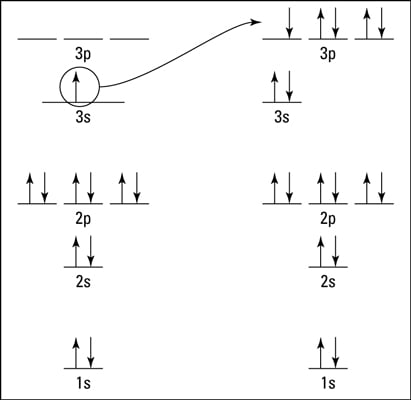 Ionic Bonds Why And How Ions Are Formed Dummies
Ionic Bonds Why And How Ions Are Formed Dummies
Ionization Energy And Electron Affinity
 Solved Create The Atomic Orbital Diagram For Chlorine En
Solved Create The Atomic Orbital Diagram For Chlorine En
 Local Density Of States Ldos Projected Onto Atomic Orbitals Upper
Local Density Of States Ldos Projected Onto Atomic Orbitals Upper
Chlorine Orbital Diagram Luxury Arrangements Of Electrons In The
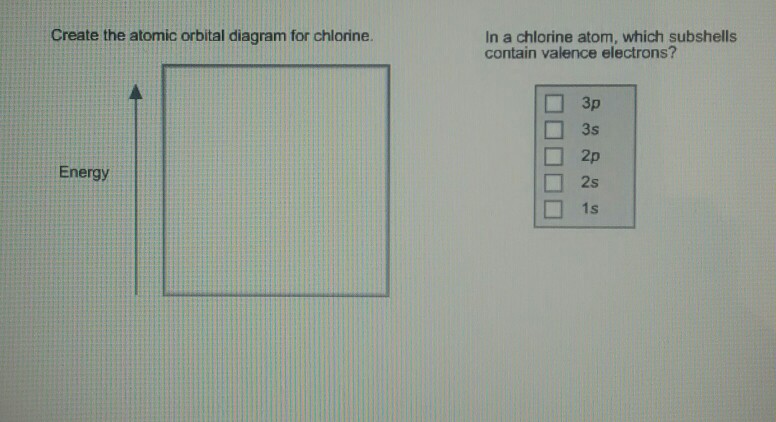
 Draw The Atomic Orbital Diagram For Chlorine
Draw The Atomic Orbital Diagram For Chlorine
 Ground State Orbital Diagram Magnesium Wiring Diagram Schematics
Ground State Orbital Diagram Magnesium Wiring Diagram Schematics
 Ground State Electron Configuration Definition Example Video
Ground State Electron Configuration Definition Example Video
 A P Dos Of Lead And Chlorine Atoms In The Aqpbcl 4 Compound And The
A P Dos Of Lead And Chlorine Atoms In The Aqpbcl 4 Compound And The
Ionization Energy And Electron Affinity
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
Chlorine Orbital Diagram Chemistry Archive September 08 2016 Air
 Chlorine Orbital Diagram Delapan Stanito Com
Chlorine Orbital Diagram Delapan Stanito Com
0 Response to "Atomic Orbital Diagram For Chlorine"
Post a Comment