Atomic Orbital Diagram For Nitrogen
Three filled bonding orbitals. Nitrogen is the seventh element with a total of 7 electrons.
How To Create An Atomic Orbital Diagram
To form 2 hybrid molecular orbitals we need to mix 2 atomic orbitals an s orbital and a p orbital.

Atomic orbital diagram for nitrogen. There are four molecular orbitals derived from the 1s and 2s orbitals. The superposition of the two 1s atomic orbitals leads to the formation of the σ and σ molecular orbitals. Start by adding the appropriate subshells.
In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. Two atomic orbitals in phase create a larger electron density which leads to the σ orbital.
Construct the orbital diagram of each atom or ion. Create the atomic orbital diagram for nitrogen. To see this video other videos chemistry education text and practice problems visit my website.
Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The remaining three electrons will go in the 2p orbital. For example boron is in the 2p block of the periodic table and so you need to show the 2p subshell and everything below it.
Many times it is necessary to see all the quantum numbers in an electron configuration this the purpose of the orbital diagram. The p orbitals combine to produce a sigma and two perpendicular pi bonds. Draw the orbital diagram for the valence shell of.
We will again need four hybrid orbitals obtained by mixing one s and three p atomic orbitals in nitrogen. If the two 1s orbitals are not in phase a node between them causes a jump in energy the σ orbital. Website is 100 free to use.
Write orbital diagram for the followingsa. In addition to listing the principle quantum number n and the subshell ell the orbital diagram shows all the different orientations and the spin of every electron. 1s2 2s2 2p3 draw two electrons for the first orbital and five for the second.
How many electrons does a fe atom have in its 3. Nitrogen has five valence electrons. Hybridization of atomic orbitals.
And three empty antibonding orbitals. Create the atomic orbital diagram for nitrogen.
Molecular Nitrogen And Related Diatomic Molecules
 I Am Very Confused About Anti Bonding Orbitals How They Form I
I Am Very Confused About Anti Bonding Orbitals How They Form I
File Atomic Orbitals Co2g Wikimedia Mons Create The Atomic Orbital
Create The Atomic Orbital Diagram For Nitrogen Inspirational Quantum
Molecular Orbital Diagram Of Oxygen Molecule Free Wiring Diagram
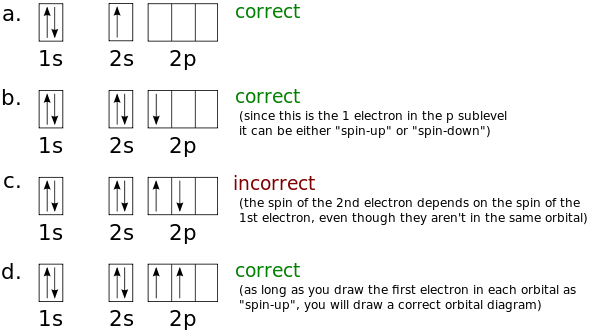 High School Chemistry Orbital Configurations Wikibooks Open Books
High School Chemistry Orbital Configurations Wikibooks Open Books
N Orbital Diagram Luxury Nitrogen Orbital Filling Diagram Nitrogen
 20 Electron Orbital Diagram For N Pictures And Ideas On Meta Networks
20 Electron Orbital Diagram For N Pictures And Ideas On Meta Networks
 Solved Create The Atomic Orbital Diagram For Nitrogen St
Solved Create The Atomic Orbital Diagram For Nitrogen St
 Nitrogen Atom Compu Ibmdatamanagement Co
Nitrogen Atom Compu Ibmdatamanagement Co
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry

Introduction To Molecular Orbital Theory
70 Elegant Stocks Of What Is The Orbital Diagram For Nitrogen Flow
Introduction To Molecular Orbital Theory
 Electron Orbital Diagram Nitrogen
Electron Orbital Diagram Nitrogen
File Orbital Diagram Nitrogen Svg Wikipedia
Energy Level Diagram For Nitrogen Best Of Describe About Electron
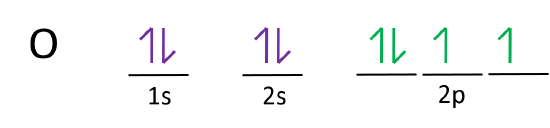 2 2 Electron Configurations Chemistry Libretexts
2 2 Electron Configurations Chemistry Libretexts
Energy Level Diagram For Nitrogen Luxury Gallery For Electron
0 Response to "Atomic Orbital Diagram For Nitrogen"
Post a Comment