Use The Mo Diagram Given To Find The Bond Order And Predict Whether H2 Exists
The bond order in sulfur dioxide for example is 15 the average of an s o single bond in one lewis structure and an so double bond in the other. In fact they do.
 Molecular Orbital Theory Ii Mo S Of The H2 Molecule Youtube
Molecular Orbital Theory Ii Mo S Of The H2 Molecule Youtube
The lewis structure for h2 is h h predicting a single bond between each hydrogen atom with two electrons in the bond.
Use the mo diagram given to find the bond order and predict whether h2 exists. The molecular orbital energy level diagram for the h2 ion. And if paramagnetic indicate the number of unpaired electrons. Based on molecular orbital theory the bond order of the h2 molecules is.
Molecular orbital diagrams of diatomic molecules. The orbital correlation diagram in predicts the same thing two electrons fill a single bonding molecular orbital. Enter the bond ord.
A single covalent bond has a bond order of one. The bond order of a diatomic molecule is defined as one half the difference between the number of electrons in bonding orbitals nb and the number of electrons in antibonding orbitals na. In molecular orbital theory we calculate bond orders by assuming that two electrons in a bonding molecular orbital contribute one net bond and that two electrons in an antibonding molecular orbital cancel the effect of one bond.
Use the mo diagram given to find the bond order and predict whether h2 exists. Qualitative mo theory orbital diagram for homonuclear diatomics. Using the mo diagram predict the bond order for t.
A triple covalent bond three and so on. Molecular orbital mo theory of the h2 molecule. A write the molecular orbital diagram as in example.
Consider how atoms come together into molecules. Enter the bond ord. You may want to reference pages 371 382section.
In its most basic form the bond order is the number of bonded electron pairs that hold two atoms together. Bond order 21 nb na figure 929. C determine if the species is diamagnetic or paramagnetic.
Example bdetermine the bond order and state whether you expect the species to be stable or unstable. 13 problem based on molecular orbital theory the bond order of the h 2 molecules is. Use the mo diagram given to find the bond order and predict whether h2 exists.
Testin g qualitative mo theory prediction of bond order with experiment for homonuclear diatomics made from elements in the 1st row of the periodic table using the molecular orbital aufbau principle. A double covalent bond a bond order of two. The following is part of a molecular orbital energ.
 Which Is More Stable He2 Or H2 And Why Quora
Which Is More Stable He2 Or H2 And Why Quora
Introduction To Molecular Orbital Theory
 3 Ways To Calculate Bond Order In Chemistry Wikihow
3 Ways To Calculate Bond Order In Chemistry Wikihow
 Chemical Bonding Molecular Orbitals Of H2 And He2 Britannica Com
Chemical Bonding Molecular Orbitals Of H2 And He2 Britannica Com
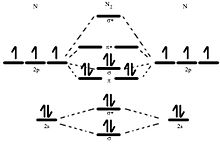 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
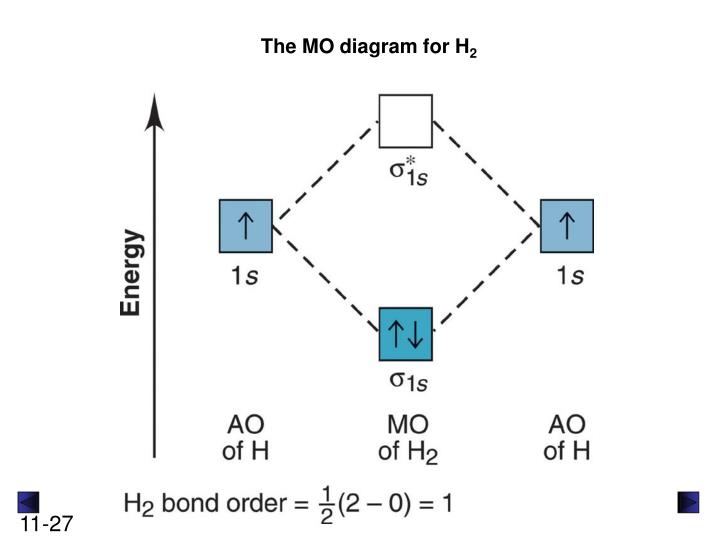 Ppt Chapter 11 Theories Of Covalent Bonding Powerpoint
Ppt Chapter 11 Theories Of Covalent Bonding Powerpoint
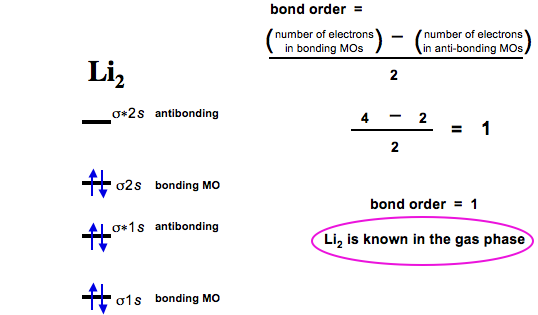 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
 Week 6 And 7 Theories Of Covalent Bonding Ppt Download
Week 6 And 7 Theories Of Covalent Bonding Ppt Download
Hybridization And Molecular Orbital Mo Theory Two Theories Of Bonding
 According To The Molecular Orbital Theory What Is The Bond Order In
According To The Molecular Orbital Theory What Is The Bond Order In
 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
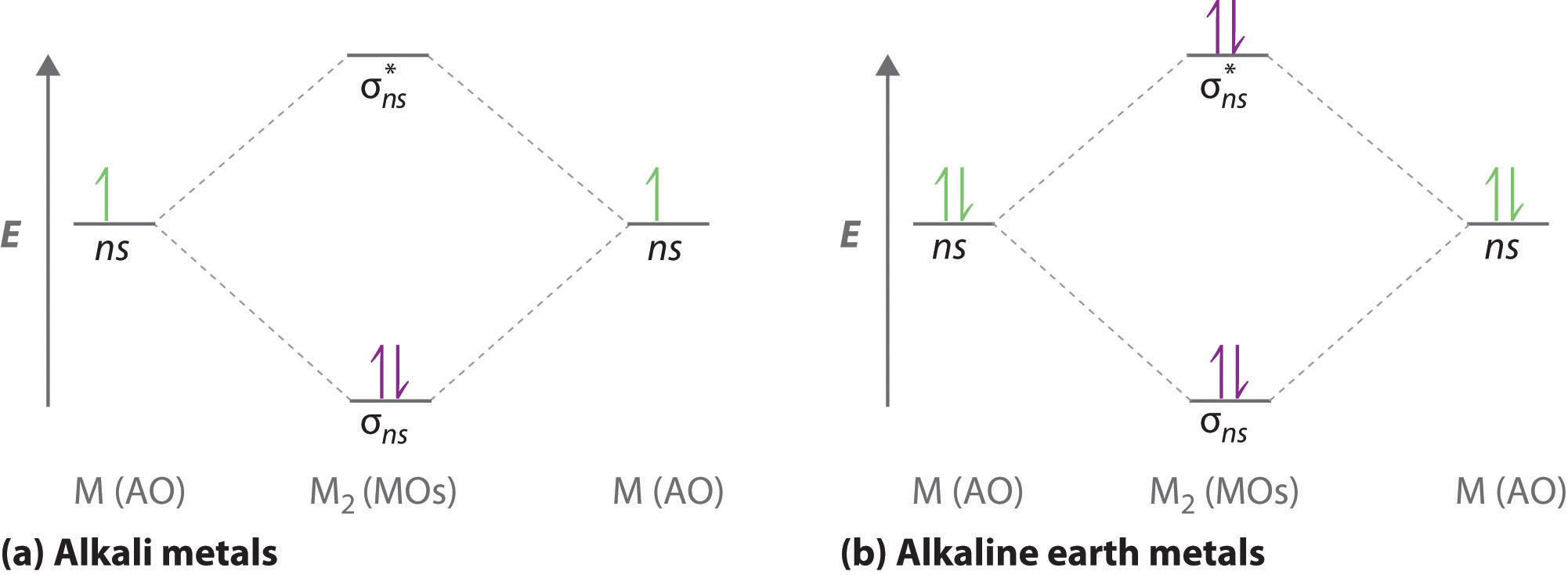 10 5 Molecular Orbital Theory Chemistry Libretexts
10 5 Molecular Orbital Theory Chemistry Libretexts
 Chemical Bonding Molecular Orbitals Of H2 And He2 Britannica Com
Chemical Bonding Molecular Orbitals Of H2 And He2 Britannica Com
Mo Diagrams For Diatomic Molecules
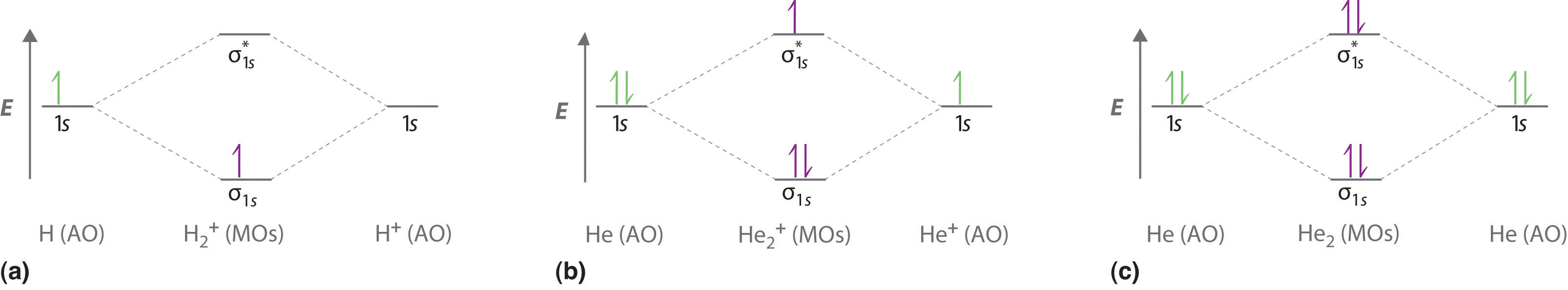 9 8 Molecular Orbital Theory Does Not Predict A Stable Diatomic
9 8 Molecular Orbital Theory Does Not Predict A Stable Diatomic
Molecular Orbital Theory Mot Chemistry Study Material
Which One Is More Stable H2 Or H2 Quora
 Chemistry 101 Molecular Orbital Theory Bond Order Bond Strength
Chemistry 101 Molecular Orbital Theory Bond Order Bond Strength
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
0 Response to "Use The Mo Diagram Given To Find The Bond Order And Predict Whether H2 Exists"
Post a Comment