How To Calculate Bond Order From Molecular Orbital Diagram
To obtain the bond order look at the molecular. In molecular orbital theory we calculate bond orders by assuming that two electrons in a bonding molecular orbital contribute one net bond and that two electrons in an antibonding molecular orbital cancel the effect of one bond.
Consider a simple example.
How to calculate bond order from molecular orbital diagram. Of electrons in anti bonding mo no. Thus the bond order is two. Molecular orbital mo theory and the bond order.
We can calculate the bond order in the o 2 molecule by noting that there are eight valence electrons in bonding molecular orbitals and four valence electrons in antibonding molecular orbitals in the electron configuration of this molecule. In molecular orbital diagram we just need to calculate the number of electrons in anti bonding orbital and bonding orbital then we can use the formula in order to calculate bond order is. In diatomic nitrogen nn for example the bond order is 3 while in acetylene hcch the bond order between the two carbon atoms is 3 and the ch bond order is 1.
In molecular orbital theory. Hydrogen atoms have one electron in the s shell. It quantifies the degree of covalent bonds between the atoms.
Bond order no. With one additional electron in an antibonding orbital 2b2 the bond order decreases by 12 relative to no. Introduction to chemistry bond order in molecular orbital theory.
Using the mo diagram of no calculate the bond order. If paramagnetism occurs due to unpaired electrons is no paramagnetic or diamagnetic. Dihydrogen h 2 with an electron in the antibonding orbital.
How to calculate bond order from molecular orbital diagram. Dihydrogen h 2 this mo diagram depicts the molecule h 2 with the contributing aos on. Of electrons in bonding mo 2.
In atoms electrons are located on atomic orbitals ao. For instance the bond order of diatomic nitrogen nn is 3 and bond order between the carbon atoms in h hc h is also three. Compare it to no.
Dihelium he 2 the third diagram hypothesizes. Know that the higher the bond order the more stable the molecule. In molecular orbital theory bond order is defined as half.
Bond order is the number of chemical bonds between a pair of atoms. Bond order indicates the stability of a bond. How to calculate bond order in chemistry know the formula.
The bond order describes the stability of the bondthe molecular orbital provides an easy understanding of the concept of the bond order of a chemical bond. Determine bond order at a.
 3 Ways To Calculate Bond Order In Chemistry Wikihow
3 Ways To Calculate Bond Order In Chemistry Wikihow
 Draw The Molecular Orbital Diagram Of Dioxygen And Calculate Bond
Draw The Molecular Orbital Diagram Of Dioxygen And Calculate Bond
What Is The Bond Order Of Co Quora
M O Diagram For B2 Chemistry Community
科学网 转载 Diatomic Species By Molecular Orbital Theory 郭令举的博文
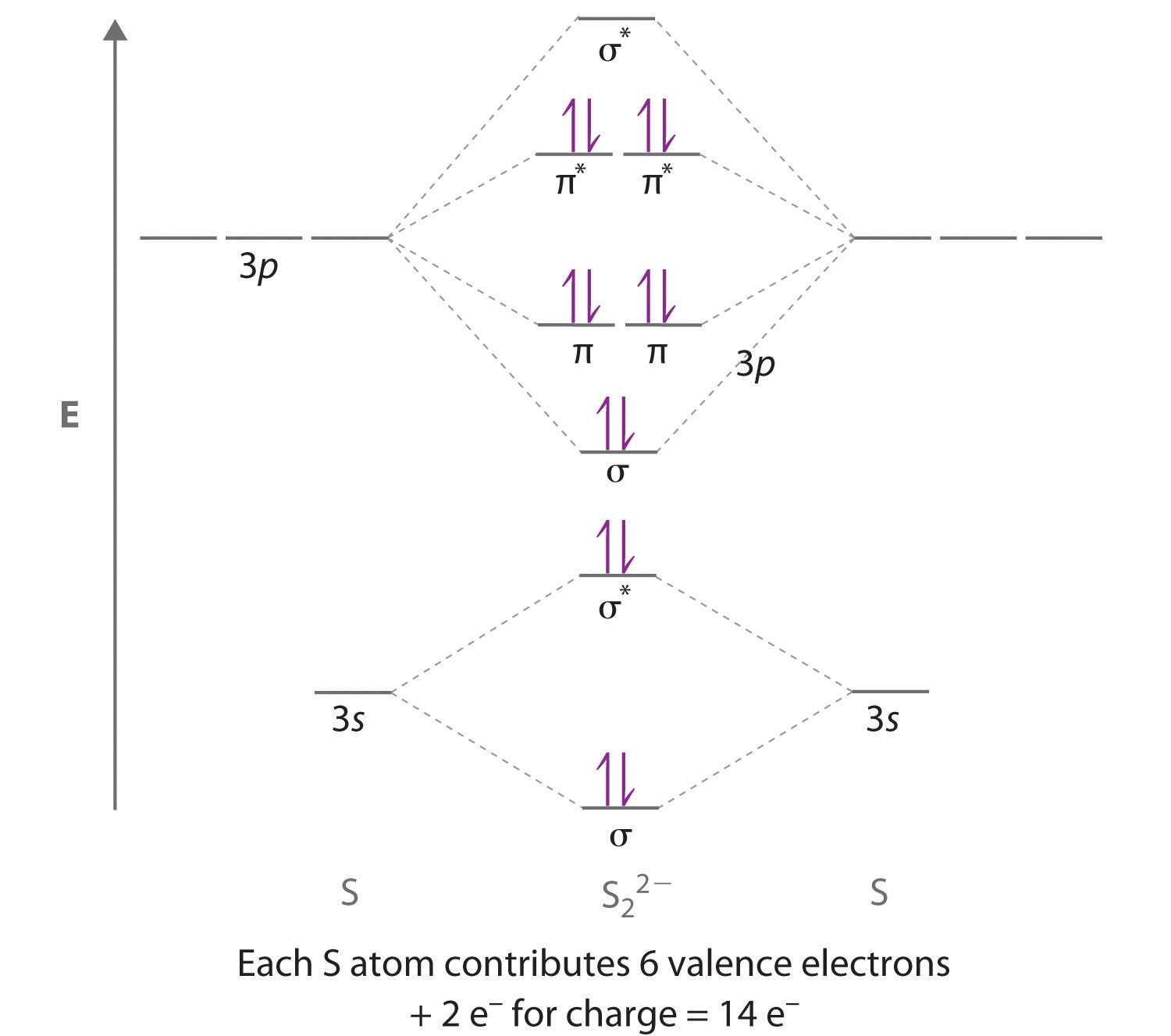 Delocalized Bonding And Molecular Orbitals
Delocalized Bonding And Molecular Orbitals
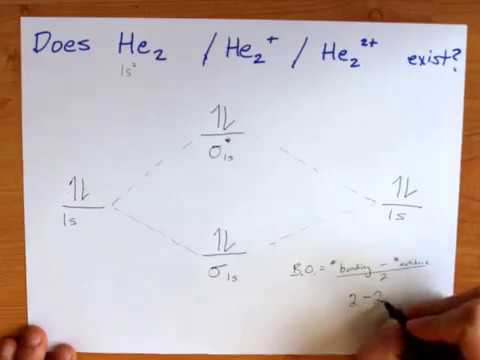 Do He2 He2 He2 2 Exist Stable Molecular Orbital Theory
Do He2 He2 He2 2 Exist Stable Molecular Orbital Theory
 Using The Mo Diagram Of No Calculate The Bond Order Compare It
Using The Mo Diagram Of No Calculate The Bond Order Compare It
Introduction To Molecular Orbital Theory
Introduction To Molecular Orbital Theory
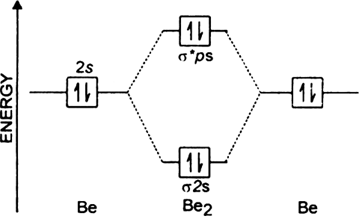 Draw The Molecular Orbital Diagram For I Be2 Ii B2 And Predict
Draw The Molecular Orbital Diagram For I Be2 Ii B2 And Predict
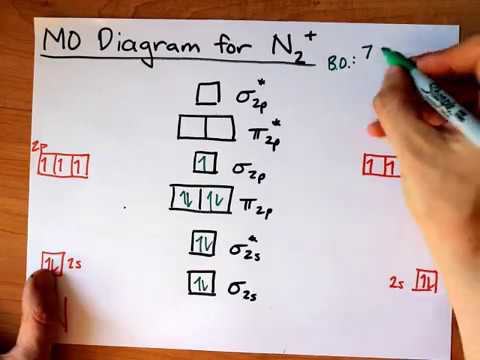 Molecular Orbital Mo Diagram Of N2 Youtube
Molecular Orbital Mo Diagram Of N2 Youtube
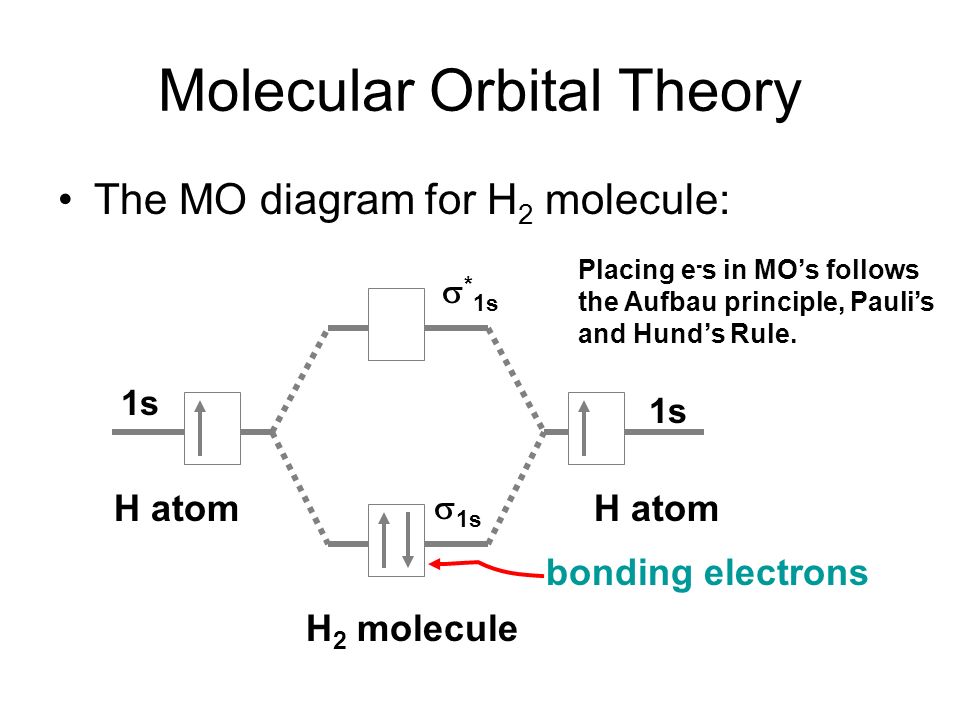 Chapter 10 Covalent Bond Theories Ppt Video Online Download
Chapter 10 Covalent Bond Theories Ppt Video Online Download
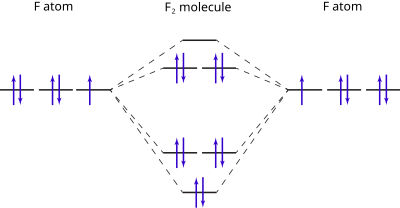 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
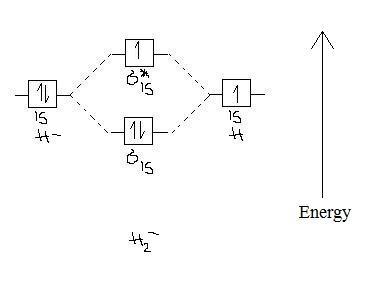 2 3b Mo Theory Of Bonding In H Chemistry Libretexts
2 3b Mo Theory Of Bonding In H Chemistry Libretexts
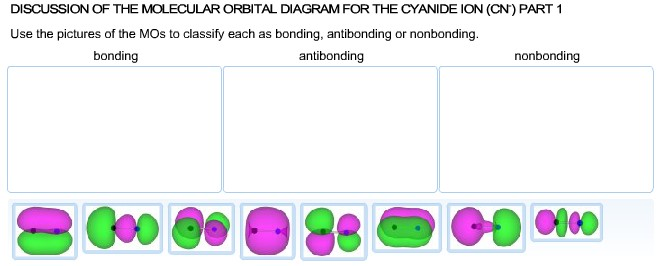 Solved Part 2 Calculate The Bond Order For Cyanide Ion P
Solved Part 2 Calculate The Bond Order For Cyanide Ion P
What Is The Molecular Orbital Diagram For Hcl Quora
0 Response to "How To Calculate Bond Order From Molecular Orbital Diagram"
Post a Comment