The Figure Is An Energy Level Diagram For A Simple Atom Figure 1
From wavelength 3 to 2. Released because the energy level of the reactants is greater than that of the products.
 How To Represent Electrons In An Energy Level Diagram Dummies
How To Represent Electrons In An Energy Level Diagram Dummies
The figure figure 1 is an energy level diagram for a simple atom.
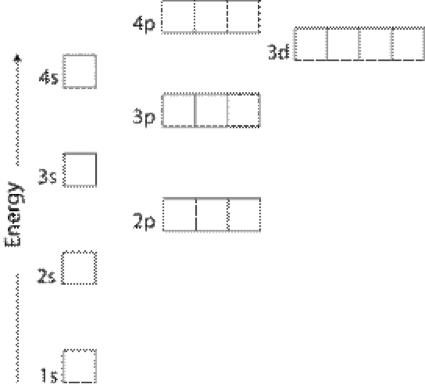
The figure is an energy level diagram for a simple atom figure 1. The figure is an energy level diagram for a quantum system. From wavelength 3 to 1. The figure is an energy level diagram for a simple atom.
The allowed energies of a simple atom are 00 ev 40 ev and 60 ev. The figure figure 1 is an energy level diagram for a simple atom. What wavelengths appear in the atoms a emission spectrum and b absorption spectrum.
Biology8 chapter 6 pratice test. I figure p298 is an energy level diagram for a simple atom. The labeled transitions a through e represent an electron moving between energy levels.
What wavelengths in nm app. The figure below represents an energy level diagram for a fictitious atom. Consider the energy diagram for a chemical reaction in figure 6 3.
The figure is an energy level diagram for a simple atom. The answer to figure p298 is an energy level diagram for a simple atom. What wavelengths appear in the systems emission spectrum.
What wavelengths appear in the atoms em. I an electron with 20 ev of figure p298 kinetic energy collides with the in class 40 ev 830 nm 500 nm 310 nm 15 ev 830 nm 310 nm 00 ev 2 only 050 ev. Answer in nm b.
What wavelengths appear in the atoms emission spectrum. Figure 1 if an electron at level 1 in a hydrogen atom absorbs 102 ev of energy it moves to level 2. What wavelengths appear in the atoms emission spectrum.
What wavelengths appear in the atoms absorption spectrum. Answer in nm 1 following. From wavelength 2 to 1.
What wavelengths appear in the atoms em. Exercise 1 description. What wavelengths appear in the atoms a emission spectrum and b absorption spectrum is broken down into a number of easy to follow steps and 24 words.
What wavelengths in nm app. Five poss election transitions are indicated labeled a through e. The following diagram represents energy levels in a hydrogen atom.
The figure is an energy level diagram for a simple atom. Overall is energy released or absorbed. The figure is an energy level diagram for a quantum system.
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
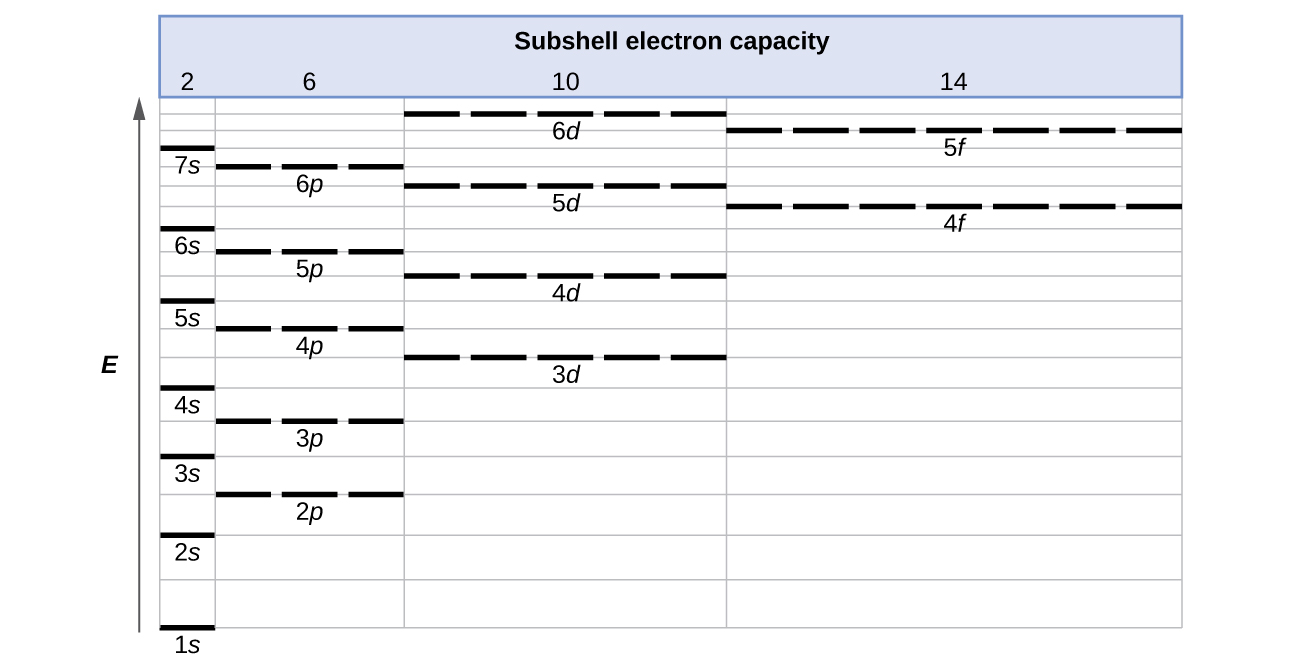 6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
Module 1 Atomic Structure Lecture 1 Structural Chemistry
 Electron Configurations Orbitals Energy Levels And Ionisation
Electron Configurations Orbitals Energy Levels And Ionisation
 Atoms What Are They What S Inside Them Explain That Stuff
Atoms What Are They What S Inside Them Explain That Stuff
 A Energy Level Diagram For The Core Excited States Of Individual O
A Energy Level Diagram For The Core Excited States Of Individual O
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 Lewis Dot Symbols And Lewis Structures Boundless Chemistry
Lewis Dot Symbols And Lewis Structures Boundless Chemistry
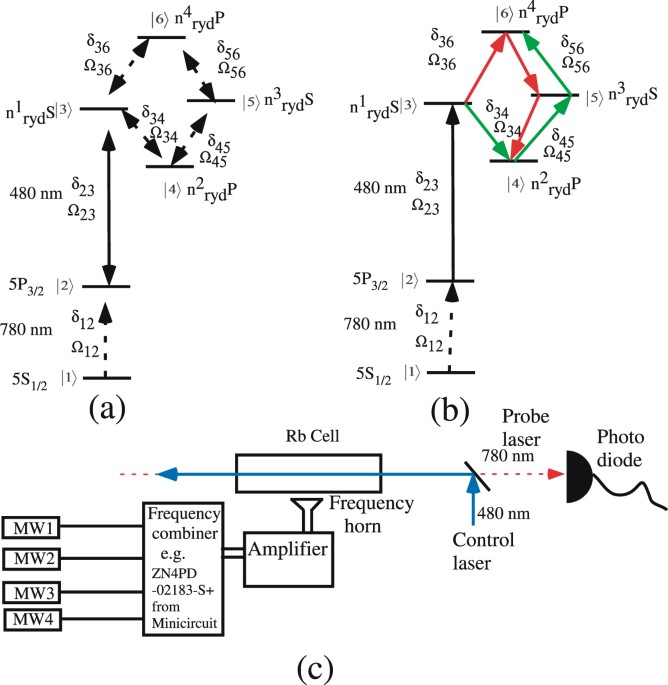 Highly Sensitive Atomic Based Mw Interferometry Scientific Reports
Highly Sensitive Atomic Based Mw Interferometry Scientific Reports
 Formation Of Spectral Lines Astronomy
Formation Of Spectral Lines Astronomy
 Chemistry I Atoms And Molecules
Chemistry I Atoms And Molecules
Drawing Atoms Montessori Muddle
Sparknotes Atomic Structure Electron Configuration And Valence
 Rydberg Interaction Induced Enhanced Excitation In Thermal Atomic
Rydberg Interaction Induced Enhanced Excitation In Thermal Atomic

 Bohr S Model Of Hydrogen Article Khan Academy
Bohr S Model Of Hydrogen Article Khan Academy
 What Is The Molecular Orbital Energy Diagram Of Co Quora
What Is The Molecular Orbital Energy Diagram Of Co Quora

0 Response to "The Figure Is An Energy Level Diagram For A Simple Atom Figure 1"
Post a Comment